Translate this page into:
Gamma-irradiated corneas for therapeutic penetrating keratoplasty, anterior lamellar keratoplasty, and sclera for glaucoma valve surgery

*Corresponding author: Rajan Sharma, MBBS, MS, Research and Clinical Associate, Cornea Centre, Sector 22C, Chandigarh U.T., India. rajansharma122@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sharma A, Sharma R, Aggarwal S, Nirankari VS. Gamma-irradiated corneas for therapeutic penetrating keratoplasty, anterior lamellar keratoplasty, and sclera for glaucoma valve surgery. IHOPE J Ophthalmol. 2024;3:3-9. doi: 10.25259/IHOPEJO_12_2022
Abstract
Objectives:
To report clinical outcomes of gamma-irradiated donor cornea (GIDC) for therapeutic penetrating keratoplasty (TPK), deep anterior lamellar keratoplasty (DALK) and gamma-irradiated donor sclera (GIDS) for glaucoma patch graft (GPG) in glaucoma filtering surgery (GFS) with Ahmed glaucoma valve (AGV).
Materials and Methods:
A prospective non-randomized, non-comparative, interventional case series of GIDC and GIDS involving 26 patients, of which 20 received GIDC (11 TPK, 9 DALK) and 6 GIDS (6 GPG). Main outcome measures were post-operative tissue characteristics as epithelialization and tissue clarity; and complications including rejection, infection, corneal melt, and other adverse effects.
Results:
Study included 26 patients, of these 20 received GIDC (11 TPK, 9 DALK) and 6 GIDS (6 GPG). Mean follow up was 8.32 ± 2.31 months. Corneal epithelium healed in 6.2 ± 2.58 days. Graft clarity was achieved in 9 (100%) DALK patients. Only 1 eye (3.8%) had a corneal melt. There were no incidences of rejection or infection in the follow up time.
Conclusion:
Gamma-irradiated sterilization of donor cornea and sclera is a new innovation to enhance utilization of donor tissue. GIDC is a promising treatment option for TPK and DALK; GIDS for GPG with good epithelialization time and tissue clarity. Gamma irradiation of both donor cornea and sclera achieved adequate sterilization and provided a long shelf life.
Keywords
Ahmad glaucoma valve
Deep anterior lamellar keratoplasty
Gamma irradiated cornea
Gamma irradiated sclera
Therapeutic penetrating keratoplasty
INTRODUCTION
The Global Vision Database has reported that 216.6 million of the world’s population suffers from moderate-to-severe vision impairment.[1] Globally, an estimated 4.9 million people (12% of the 39 million total blind population) suffer from bilateral corneal blindness.[2] Further global breakdown shows a heavy burden of corneal blindness on the developing world, as 98% of corneal blindness affects developing countries.[2] In addition, 1.5–2.0 million new cases of monocular blindness are being added every year.[3] Infectious keratitis resulting from corneal trauma is the most common cause.[4] While a significant proportion of corneal blindness is avoidable by instituting effective primary preventive measures at the community level for conditions such as Vitamin A deficiency, trachoma, onchocerciasis, and ophthalmia neonatorum. Timely and prompt medical management can help to reduce subsequent visual impairment in cases of corneal ulceration. However, due to a lack of resources, many cases progress to result in severe visual impairment and require strategies for visual rehabilitation by corneal transplantation.[5] Penetrating keratoplasty (PKP/PK) and deep anterior lamellar keratoplasty (DALK) are the common types of corneal transplantation worldwide, whereas the trend is increasing toward endothelial keratoplasty in Western countries.
Corneal surgeons in developing countries face an acute shortage of good quality donor cornea. A recent global survey of corneal transplant facilities in 148 countries reveals that a majority of the world’s population has no access to corneal transplantation. An average of one cornea is available for 70 patients needing corneal transplants.[6] In the developing world, procurement of donor cornea remains low due to a lack of awareness and cultural barriers.[7,8] Even though the procurement rate of the donor tissue has recently increased, the overall utilization rate remains low (about 65%).[6] Due to the non-availability of good quality optical grade donor tissue and lack of access to long-term storage media, the utilization rate of donor cornea is low in the developing world.[9] These issues have prompted the evaluation of alternative methods of storage to prolong tissue viability and increase tissue supply for corneal transplantation. Some authors have recommended the use of glycerin-preserved tissue, but this tissue requires rehydration, which makes it difficult to handle intraoperatively due to increased thickness and haze. In addition, glycerin-preserved donor cornea may require several weeks to clear.[10,11]
Another option is gamma-irradiated tissue which is a clear tissue option that does not require rehydration.[12-14] Reports have also shown that gamma-irradiation does not alter collagen structure, fibril diameter, and interfibrillar distance.[6] Gamma-irradiation reduces the allogenicity of the corneas, potentially reducing the risk of graft rejection.[11,15] Compared to glycerin-preserved corneal tissue, gamma-irradiation offers a safer therapeutic option because it sterilizes against bacteria, fungi, viruses, and prions.[15-17] Surgeons report a similar ease of use and tensile strength intraoperatively when compared to the standard fresh corneal tissue.[13]
Because of these reasons, we evaluated the safety and clinical efficacy of gamma-irradiated donor cornea (GIDC) in performing therapeutic penetrating keratoplasty (TPK) and deep anterior lamellar keratoplasty (DALK); and gamma-irradiated donor sclera (GIDS) in glaucoma filtering surgery (GFS) with Ahmed glaucoma valve (AGV).
MATERIALS AND METHODS
This prospective, non-comparative, interventional case series, included 26 patients (22 males and 4 females), who underwent TPK (11 patients), deep anterior lamellar keratoplasty (9 patients) using GIDC, and glaucoma filtering surgery with AGV (6 patients) using GIDS. This study was approved by the institutional review board. The study has been conducted with strict adherence to the tenets of the Declaration of Helsinki. The indications for TPK were infectious keratitis not responding to medical treatment or corneal perforations larger than 3.0 mm in diameter. DALK was considered for patients having corneal scars involving the anterior half of the cornea and advanced keratoconus. GFS with AGV was performed in patients with post-PK glaucoma not controlled with maximum medical therapy. Patients suffering from ocular surface disease, dry eye, or systemic autoimmune disorders such as rheumatoid arthritis/Wegner’s granulomatosis were excluded from the study. All patients underwent complete ophthalmological examination, including slit-lamp biomicroscopy and Siedel’s test to confirm the presence of a corneal perforation. Detailed microbiological tests including direct microscopy of Gram-stained smears/KOH wet mount preparation and bacterial/fungal cultures of the material obtained on corneal scrapings were performed. Primary outcome measures were the clinical efficacy of GIDC and GIDS. Secondary outcome measures included tissue characteristics such as epithelialization, tissue clarity, and complications including corneal melt, rejection, infection, and other adverse effects.
Gamma-irradiated tissue preparation
Donor corneas were procured using aseptic measures and standard eye banking guidelines. The donors were serologically screened for infectious diseases, including human immunodeficiency virus, hepatitis B, hepatitis C, and syphilis. Donor eyes were disinfected by immersing the whole globes in a povidone-iodine (5%) solution for 5 min. The whole globes were then rinsed with normal saline for 10 min. Excision of the donor cornea with the sclera rim (2.0 mm) was done under laminar flow. The corneoscleral rims were stored in a McCarey-Kaufman (MK) medium for slit-lamp biomicroscopy and specular microscopy. The donor corneas not suitable for optical penetrating keratoplasties were selected for gamma irradiation. After the excision of the donor cornea with the scleral rim (2 mm), the contents of the eyeball including the vitreous, retina, iris, ciliary body, and choroid were scooped out. The scleral shell was cleared off any remnants of the uveal tissue. Four pieces of the sclera in between the recti insertions were cut and stored in MK medium. Donor corneas were removed from the MK medium and were put into a storage medium containing human serum albumin. The scleral patches were air-dried in the laminar flow and sterilized using gamma irradiation (Biocover Lab, Karnal, India). Radiation sterilization at a dose of 25 kGy of gamma radiation recommended for the sterilization of medical products, including tissue allografts was used.[18,19] The dose of radiation 25 kGy for sterilization was proposed as it is 40% above the minimum dose required to kill the resistant microorganisms.[19] These GIDCs are approved to be stored in the human serum albumin at room temperature for up to 2 years. The GIDS may be stored for up to 2–5 years.
Surgical technique
TPK and deep anterior lamellar keratoplasty were performed using GIDC and glaucoma filtering surgery with AGV using GIDS.
The eye was draped and prepared under sterile measures. The GIDC and GIDS tissues were removed from the sterile vial and rinsed with a balanced salt solution before use.
TPK
Eleven patients underwent TPK with GIDC. The maximum diameter of the diseased cornea (size of infiltrate/corneal perforation) was measured to determine the size of host trephination. The host corneal trephination was performed with a handheld disposable trephine. All necessary precautions were taken not disturb the intraocular contents during trephination. The GIDC was oversized by 0.5 mm in all cases. The donor cornea size varied between 7.5 and 8.5 mm in diameter. The GIDC was secured in the recipient cornea using 16 interrupted 10-0 nylon sutures.
A total of nine patients (nine eyes) underwent DALK with GIDC. The surgery was performed under peribulbar anesthesia using the manual technique. In three patients with keratoconus, the same size graft was used. For the rest of patients with other etiologies, the graft was oversized by 0.5 mm. The donor size varied between 8.0 and 9.0 mm in diameter. Trypan blue was applied after trephination to ensure the complete removal of the Descemet’s membrane. The graft was secured in the recipient cornea using either 16 interrupted 10-0 nylon sutures.
Glaucoma filtering surgery with AGV
Fornix-based conjunctival incision was given in the superior temporal area. AGV was primed with balanced salt solution with 27G cannula. Tenon’s capsule was dissected from episcleral tissue, and light cautery was done to episcleral vessels. Body implant is secured 8–10 mm posterior to the limbus. The plate is anchored to the sclera with a 10–0 nylon suture. The drainage tube is trimmed to allow a 2–3 mm insertion into the anterior chamber (AC). The AC was then entered 1–3 mm posteriorly to the corneoscleral limbus with a 22 gauge disposable needle. The tube is inserted into the AC through the needle tract. Care is taken to ensure that the drainage tube does not contact iris or corneal endothelium after insertion. The AGV tube was fixed to the sclera with a 10-0 nylon suture.
To cover the AGV tube, a 4.0 × 4.0 mm rectangular GIDS patch was cut. A GIDS patch was implanted over the AGV tube and sutured to the sclera with several 10-0 nylon sutures. The GIDS patch was covered with the conjunctiva by suturing it to the limbus with 7-0 Vicryl sutures.
The remaining GIDC and GIDS tissues were sent for culture according to the cornea transplantation protocol after replacing it into the storage medium.
RESULTS
Of the 26 patients, 22 (85%) were male and 4 (15%) female. The mean age of the patients was 43.46 (range 17–72) years. Eleven patients underwent TPK, 9 DALK, and 6 GFS with AGV. TPK was performed to treat perforated fungal ulcers (5), bacterial corneal ulcers (4), and recurrent herpes simplex keratitis (2). DALK was undertaken for advanced keratoconus (3), corneal opacity (3), descemetocele (2), and multiple intrastromal foreign bodies (1). Glaucoma filtering surgery with AGV was done for patients with uncontrolled glaucoma despite maximal topical ocular hypotensive therapy post-PK (5 primary PK and 1 repeat PK). The details of the clinical characteristics of all the patients belonging to the three groups are shown in [Tables 1-3]. The mean follow-up was 8.32 ± 2.31 months. The corneal epithelium for the TPK and DALK group healed within a mean of 6.2 ± 2.58 days. TPK with GIDC was successful in eradicating infection and restoring integrity in all 11 (100%) patients. A fungal corneal ulcer that healed successfully following TPK with GIDC is shown in Figures 1a and b. The post-operative visual acuity in the TPK group ranged from HM to 2/60. Graft clarity was achieved in 8 (90.9%) patients following primary DALK and in 1 (9.1%) after resurgery with GIDC. The figures 1c and d show a clear graft following DALK with GIDC in a patient with corneal opacity. None of our patients needed surgical revision in this group. None of the patients in this group developed graft rejection or endothelial failure. Visual acuity in patients who had undergone AGV ranged from 3/60 to 6/12 [Figures 1 e-f]. Graft melt occurred in 1/9 (9.1%) patients at 3 weeks post-operative in the TPK group. The primary indication of TPK in this patient was a non-healing corneal ulcer due to herpes simplex virus. The patient was successfully regrafted with GIDC. Patients in the DALK group achieved visual acuity ranging from 6/36 to 6/12. None of the patients in the TPK and DALK groups developed graft rejection. The mean pre-operative IOP in the AGV group was 45.6 ± 7.02 mmHg compared to 16.5 ±1.71 mmHg post-operatively. We did not observe conjunctiva necrosis, necrosis of sclera patch, and signs suggestive of sclera patch inflammation in any of GFS with AGV patients. None of our patients needed surgical revision in this group. None of the patients in this group developed graft rejection or endothelial failure. Visual acuity in patients who had undergone AGV ranged from 3/60 to 6/12.
| S. No. | Age | Sex | Indications | Type of surgery | Size of recipient/donor trephination | Graft surface healing | Complication | Graft status | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|
| 1. | 38 | Male | Fungal corneal ulcer | TPK | 8.5×9.0 mm | 5 | Nil | Opaque | 11.0 |
| 2. | 53 | Male | Fungal corneal ulcer | TPK | 7.5×8.0 mm | 7 | Increased exudates | Opaque | 08.0 |
| 3. | 24 | Male | Fungal corneal ulcer | TPK | 7.5×8.0 mm | 6 | Nil | Opaque | 10.0 |
| 4. | 59 | Male | Fungal corneal ulcer | TPK | 8.5×9.0 mm | 4 | Nil | Opaque | 06.0 |
| 5. | 29 | Female | Fungal corneal ulcer | TPK | 8.5×9.0 mm | 8 | Increase exudates | Opaque | 09.0 |
| 6. | 57 | Male | Bacterial corneal ulcer | TPK | 6.0×6.5 mm | 3 | Nil | Opaque | 05.5 |
| 7. | 26 | Male | Bacterial corneal ulcer | TPK | 7.5×8.0 mm | 5 | Nil | Opaque | 10.5 |
| 8. | 23 | Male | Bacterial corneal ulcer | TPK | 7.0×7.5 mm | 9 | Nil | Opaque | 07.0 |
| 9. | 47 | Male | Bacterial corneal ulcer | TPK | 6.5×7.0 mm | 7 | Nil | Opaque | 08.5 |
| 10. | 39 | Male | Herpes simplex keratitis | TPK | 7.5×8.0 mm | 5 | Nil | Opaque | 07.5 |
| 11. | 41 | Male | Herpes simplex keratitis | TPK | 8.0×8.5 mm | 6 | Nil | Opaque | 06.5 |
TPK: Therapeutic penetrating keratoplasty, GIDC: Gamma-irradiated donor corneas
| S. No. | Age | Sex | Indications | Type of surgery | Size of recipient/donor trephination | Post-op graft surface healing (days) | Complication | Graft status | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|
| 1. | 67 | Male | Climatic droplet keratopathy | DALK | 7.5×8.0 mm | 5 | Nil | Clear | 11.0 |
| 2. | 49 | Male | Corneal opacity | DALK | 7.5×8.0 mm | 6 | Nil | Clear | 14.0 |
| 3. | 58 | Female | Corneal opacity | DALK | 7.5×8.0 mm | 8 | Nil | Clear | 05.5 |
| 4. | 26 | Female | Keratoconus | DALK | 7.5×8.0 mm | 4 | Nil | Clear | 09.5 |
| 5. | 19 | Male | Keratoconus | DALK | 7.5×8.0 mm | 3 | Nil | Clear | 07.0 |
| 6. | 57 | Male | Keratoconus | DALK | 7.5×8.0 mm | 4 | Nil | Clear | 10.5 |
| 7. | 17 | Male | Multiple stromal foreign bodies | DALK | 7.5×8.0 mm | 5 | Nil | Clear | 06.5 |
| 8. | 61 | Male | HSK, Descemetocele | DALK | 7.5×8.0 mm | 11 | Prolonged epithelial healing | Clear | 05.5 |
| 9. | 43 | Male | HSK, Descemetocele | DALK | 7.5×8.0 mm | 13 | Graft melt, Regraft | Opaque | 09.5 |
GIDC: Gamma-irradiated donor corneas, HSK: Herpes simplex keratitis, DALK: Deep anterior lamellar keratoplasty
| S. No. | Age | Sex | Indication for PKP | Indication for GFS with AGV | Size of GIDS | Pre-op/Post-op IOP | Graft status | Complication | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|
| 1. | 38 | Male | Traumatic corneal opacity | Post PK glaucoma | 4.0×4.0 mm | 42/16 | Clear | Nil | 12.0 |
| 2. | 72 | Male | Opaque graft | Post PK glaucoma | 4.0×4.0mm | 48/17 | Clear | Nil | 07.0 |
| 3. | 24 | Male | Adherent leukoma | Post PK glaucoma | 3.5×3.5mm | 39/15 | Clear | Nil | 09.0 |
| 4. | 59 | Male | Corneal opacity with glaucoma | Post PK glaucoma | 4.0×4.0 mm | 37/14 | Clear | Nil | 11.5 |
| 5. | 47 | Female | Adherent leukoma with glaucoma | Post PK glaucoma | 4.0×4.0 mm | 51/18 | Opaque | Nil | 06.0 |
| 6. | 57 | Male | Traumatic corneal scar | Post PK glaucoma | 4.0×4.0 mm | 57/19 | Clear | Nil | 07.5 |
AGV: Ahmed glaucoma valve, PKP/PK: Penetrating keratoplasty, GIDS: Gamma irradiated donor sclera, IOP: Intraocular pressure, GFS: Glaucoma filtering surgery
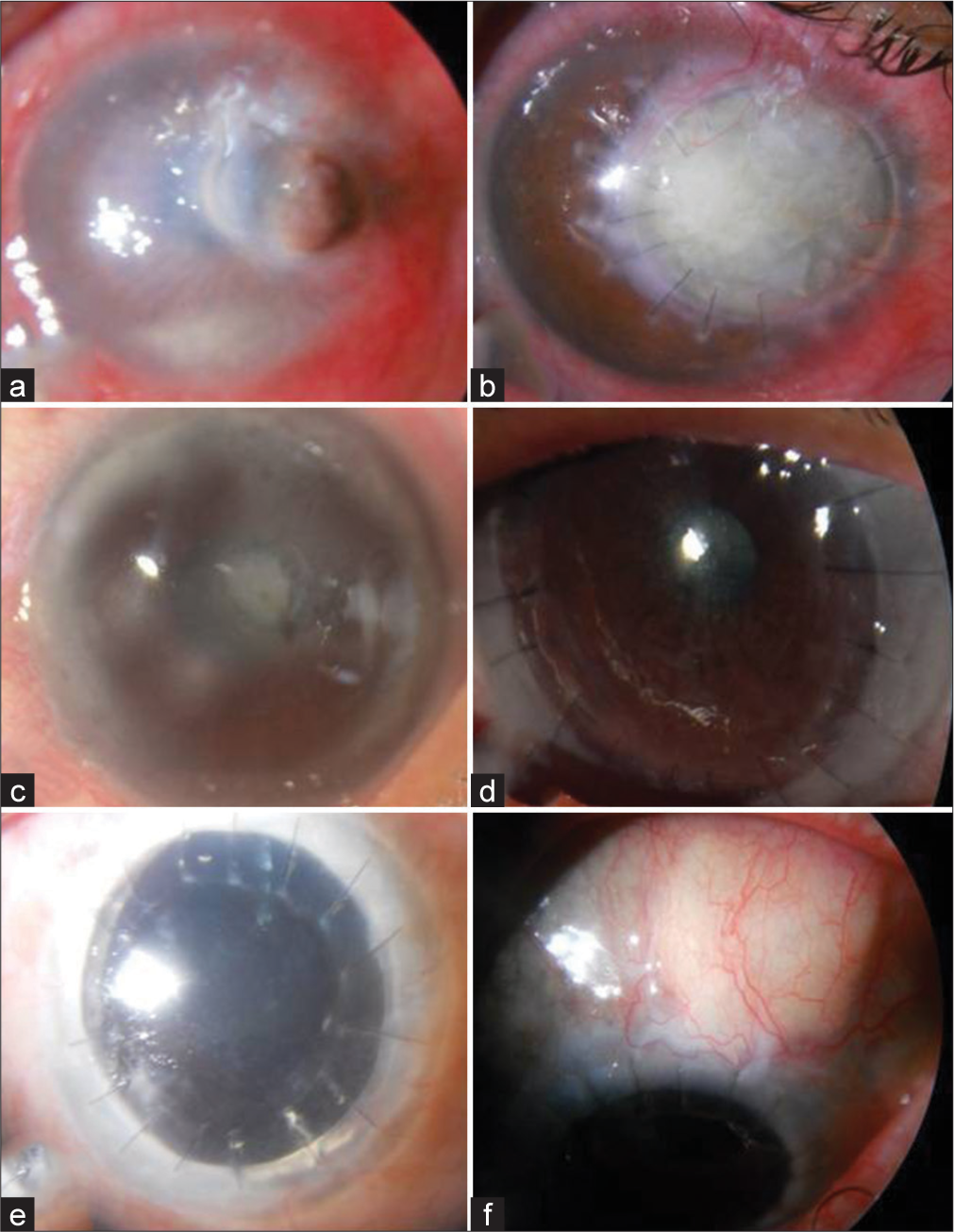
- (a) Perforated fungal corneal ulcer (b) Opaque graft post-therapeutic penetrating keratoplasty using Gamma-irradiated donor corneas (GIDC) at 12 weeks (c) Corneal opacity pre-operative (d) Clear graft post-Deep anterior lamellar keratoplasty (DALK) using GIDC at 4.5 months (e). Post-penetrating keratoplasty (Post-PK) glaucoma pre-operative (f) GFS with Ahmed glaucoma valve using gamma-irradiated donor sclera at 3 months.
DISCUSSION
Our study demonstrates the successful use of GIDC for PKP and DALK and GIDS for GFS with AGV. We did not encounter infection, unusual inflammation, or any other complications.
In developing countries, there is always a shortage of donor corneas has been observed. To make donor tissue available at short notice is extremely difficult in the developing world. Ensuring sterilization of the donor tissue is another Herculean task for the eye banks. A high prevalence of gentamycin resistance in corneal preservation media (Optisol-GS and Eusol-C) has been reported.[20] The bacteria resistant to gentamycin has been found susceptible to vancomycin.[20] The role of antifungal agents such as amphotericin and voriconazole in Optisol-GS is being evaluated.[21] In contrast, gamma sterilization of tissue allografts is effective against bacteria, fungi, viruses, and prions. Radiation sterilization ensures a high safety factor that even tests for sterility are generally considered unwarranted.[18,19]
Gamma-irradiated cornea/sclera is a good alternative to fresh donor tissue. GIDC/GIDS can be stored at room temperature, has a long shelf life, and can be made available at short notice. Gamma irradiation does cause a loss of keratocytes and endothelial cells and strengthens structural integrity.[22] In addition, it preserves the hydration, light transmittance, and elasticity of the tissue comparable to the fresh corneas.[22] Due to the good light transmittance with partial and full-thickness gamma-irradiated lenticules,[19] this tissue demonstrates optimal optical outcomes when used in lamellar corneal transplantation. Due to the lack of viable endothelial cells, GIDC cannot be used for optical PKP. However, GIDC can be successfully used for tectonic PK. Further, gamma irradiation results in reduced allogenicity allogenicity of the graft as demonstrated by decreased expression of T-helper cell-associated cytokines, absence of secondary alloimmune response of T-cells, and delayed hypersensitivity response in graft recipients of GIDC tissue.[15] This suggests that risk of immune rejection may be lowered with use of GIDC. GIDC and GIDS have long shelf life, can be stored at room temperature, and can be easily transported when needed.
In our study, we did not encounter any GIDC or GIDS-related complications. The use of GIDC and GIDS in our experience is safe for clinical use. GIDC has been used successfully by several authors. A study reported the successful use of GIDC in lamellar keratoplasty (10 patients). These authors reported no incidence of immune rejection, infection, opacification, or neovascularization during 7–15-month follow-up.[12] These authors recently reported long-term clinical outcomes of partial and full thickness keratoplasty using GIDC in 23 patients. In this study, in nine of the patients who had surgery for non-inflammatory conditions, GIDC stayed intact for 24 months and 7 out of 9 remained optically clear at 12-month follow-up. In cases where keratoplasty was done for infectious keratitis, the majority remained intact for a median of 30 months; however, one-third had recurrent infectious keratitis in the 1–3 months of the post-operative period.[23] In patients undergoing DALK with a history of intractable ocular surface diseases, amniotic membrane transplantation was used in conjunction with DALK. Surface re-epithelialization was seen in all cases within 2 weeks and most grafts remained clear without rejection or opacification.[24] Sclera, pericardium, cornea, and dura mater are the commonly used patch graft materials in glaucoma drainage device surgery (GDDS).[25] In a comparative study, sclera, pericardium, and cornea patches were found equally effective in GDDS.[26] We did not have erosion of the tube in any of our patients. GIDS appears to be a viable alternative to radiation-sterilized pericardium in GDDS.
GIDC has also been reported to be used successfully in lieu of fresh corneal tissue as a carrier for Boston type 1 keratoprosthesis.[27] A study evaluated the outcome of GIDC to fresh corneal tissue with and without endothelium and cryopreserved cornea in a rabbit model and found that, while GIDC remains clearer and thinner than cryopreserved cornea and fresh cornea without endothelium but inferior to fresh cornea with endothelium and cannot be used for full-thickness keratoplasty.[28] Several authors have used GIDC successfully to cover glaucoma implants and shown satisfactory tube coverage without erosion and tissue transparency over the post-operative period.[29,30] However, the graft thickness is shown to decrease over time after the surgery,[31] but there is no increased incidence of tube exposure when covered by GIDC compared to traditional scleral patch grafts.[32] Electron beam irradiation has also been used to store and sterilize donor cornea and sclera.[33] No increase in erosion has been reported with the use of electron beam-irradiated donor sclera compared to GIDS following GFS using GDD.[33]
Despite the advantages of using GIDC, there are significant limitations. This tissue cannot be used for full-thickness keratoplasty. Studies on long-term outcomes of the use of GIDC and GIDS are relatively limited. This calls for continued research in the area of corneal transplantation to develop cost-effective efficient alternatives to fresh donor corneal tissue. Guidelines are being developed for the use of human donated tissue for corneal transplantation, research, and future technologies, as outlined in the Barcelona principles.[34] Recent research studies show an effort to develop xenogeneic corneas using sterilization methods such as gamma irradiation[35] and X-rays.[36]
Limitations of this study include the small number of patients and lack of follow-up data. In addition, other objective corneal findings would be helpful, such as specular biomicroscopy, automated topography, and confocal biomicroscopy, particularly in patients who underwent optical procedures. Nevertheless, we believe our results are an important addition to the current scope of knowledge and will hopefully encourage greater utilization of this corneal tissue.
There has been an acute shortage of donor corneas during COVID-19. Although elective corneal transplant procedures were discontinued during the pandemic crisis, emergency procedures such as therapeutic keratoplasty were being performed at tertiary care units. Gupta et al. found glycerol preserved corneas (GPC) comparable to fresh cornea tissue in therapeutic keratoplasty.[37] GIDC and sclera tissue may also be useful in performing emergency procedures;[38] however, gamma-sterilized corneas need to be compared with GPC in therapeutic keratoplasty.
CONCLUSION
Reports on measures to enhance donor corneas utilization in developing countries are lacking. There are several reports on the shortage of donor corneas in developing countries. Gamma irradiated sterile cornea (GISC) is another option for compensating the limited availability of donor cornea in developing world especially in procedures not requiring high endothelial count. Hence, GIDC and GIDS offer valuable treatment options for various interventions, including therapeutic penetrating keratoplasty, deep anterior lamellar keratectomy, and GDDS and should be considered as an initial treatment option, especially in times and areas of limited donor tissue availability.
Ethical approval
The author(s) declare that they have taken the ethical approval from IRB (No. 0006/21).
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Vision loss expert group of the global burden of disease study. Global causes of blindness and distance vision impairment 1990-2020: A systematic review and meta-analysis. Lancet Glob Health. 2017;5:e1221-34.
- [Google Scholar]
- Turning the tide of corneal blindness. Indian J Ophthalmol. 2012;60:423-7.
- [CrossRef] [PubMed] [Google Scholar]
- Burden of corneal blindness in India. Indian J Community Med. 2013;38:198-206.
- [CrossRef] [PubMed] [Google Scholar]
- Global survey of corneal transplantation and eye banking. JAMA Ophthalmol. 2016;134:167-73.
- [CrossRef] [PubMed] [Google Scholar]
- Corneal donations in South Africa: A 15-year review. S Afr Med J. 2017;107:697-701.
- [CrossRef] [PubMed] [Google Scholar]
- Awareness and attitudes toward corneal donation: Challenges and opportunities. Clin Ophthalmol. 2018;12:1049-59.
- [CrossRef] [PubMed] [Google Scholar]
- Procurement, storage and utilization trends of eye banks in India. Indian J Ophthalmol. 2019;67:1056-9.
- [CrossRef] [PubMed] [Google Scholar]
- The use of glycerol-preserved corneas in the developing world. Middle East Afr J Ophthalmol. 2010;17:38-43.
- [Google Scholar]
- Comparison of fresh corneal tissue versus glycerincryopreserved corneal tissue in deep anterior lamellar keratoplasty. Invest Ophthalmol Vis Sci. 2010;51:775-81.
- [CrossRef] [PubMed] [Google Scholar]
- Lamellar keratoplasty using gamma-irradiated corneal lenticules. Am J Ophthalmol. 2011;151:170-4.e1.
- [CrossRef] [PubMed] [Google Scholar]
- The intraoperative impression and postoperative outcomes of gamma-irradiated corneas in corneal and glaucoma patch surgery. Cornea. 2011;30:1387-91.
- [CrossRef] [PubMed] [Google Scholar]
- The use of precut γ-irradiated corneal lenticules in Boston Type 1 keratoprosthesis implantation. Am J Ophthalmol. 2012;154:495-8.
- [CrossRef] [PubMed] [Google Scholar]
- Gamma-irradiation reduces the allogenicity of donor corneas. Invest Ophthalmol Vis Sci. 2012;53:7151-8.
- [CrossRef] [PubMed] [Google Scholar]
- Risk of prion disease transmission from ocular donor tissue transplantation. Cornea. 1999;18:2-11.
- [CrossRef] [PubMed] [Google Scholar]
- Inactivation of viral and prion pathogens by gamma-irradiation under conditions that maintain the integrity of human albumin. Vox Sang. 2003;84:36-44.
- [CrossRef] [PubMed] [Google Scholar]
- Radiation sterilization of tissue allografts: A review. World J Radiol. 2016;8:355-69.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of broadband spectral transmission characteristics of fresh and gamma-irradiated corneal tissues. Cornea. 2015;34:228-34.
- [CrossRef] [PubMed] [Google Scholar]
- In vitro susceptibility of microorganisms isolated from cold stored corneas: Increased gentamicin-resistance in cornea banking. Cell Tissue Bank. 2020;21:159-65.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of voriconazole as an additive in Optisol GS: A preservation medium for corneal donor tissue. Cornea. 2007;26:343-7.
- [CrossRef] [PubMed] [Google Scholar]
- Physical and biological characterization of the gamma-irradiated human cornea. Cornea. 2015;34:1287-94.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term clinical outcomes of keratoplasty using gamma-irradiated corneal lenticules. BMJ Open Ophthalmol. 2019;4:e000396.
- [CrossRef] [PubMed] [Google Scholar]
- Deep anterior lamellar keratoplasty using irradiated acellular cornea with amniotic membrane transplantation for intractable ocular surface diseases. Korean J Ophthalmol. 2015;29:79-85.
- [CrossRef] [PubMed] [Google Scholar]
- Grafts in glaucoma surgery: A review of the literature. Asia Pac J Ophthalmol (Phila). 2017;6:469-76.
- [Google Scholar]
- Donor sclera versus bovine pericardium as patch graft material in glaucoma implant surgery and the impact of a drainage suture. Acta Ophthalmol. 2018;96:692-8.
- [CrossRef] [PubMed] [Google Scholar]
- Gamma-irradiated corneas as carriers for the Boston Type 1 keratoprosthesis: Advantages and outcomes in a surgical mission setting. Cornea. 2014;33:235-9.
- [CrossRef] [PubMed] [Google Scholar]
- Gamma-irradiated sterile cornea for use in corneal transplants in a rabbit model. Middle East Afr J Ophthalmol. 2015;22:346-51.
- [CrossRef] [PubMed] [Google Scholar]
- Gamma-irradiated cornea allograft for glaucoma surgery. J Glaucoma. 2013;22:355-7.
- [CrossRef] [PubMed] [Google Scholar]
- Tube shunt coverage with gamma-irradiated cornea allograft (VisionGraft) Clin Ophthalmol. 2015;9:751-5.
- [CrossRef] [PubMed] [Google Scholar]
- Measurement of gamma-irradiated corneal patch graft thickness after aqueous drainage device surgery. JAMA Ophthalmol. 2017;135:941-6.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical outcomes of gamma-irradiated sterile cornea in aqueous drainage device surgery: A multicenter retrospective study. Eye (Lond). 2017;31:430-6.
- [CrossRef] [PubMed] [Google Scholar]
- Electron beam irradiated corneal versus gamma-irradiated scleral patch graft erosion rates in glaucoma drainage device surgery. Ophthalmol Ther. 2019;8:421-6.
- [CrossRef] [PubMed] [Google Scholar]
- The Barcelona principles: An agreement on the use of human donated tissue for ocular transplantation, research, and future technologies©. Cornea. 2018;37:1213-7.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of gamma radiation sterilization on the structural and biological properties of decellularized corneal xenografts. Acta Biomater. 2019;96:330-44.
- [CrossRef] [PubMed] [Google Scholar]
- Small incision femtosecond laser-assisted X-ray-irradiated corneal intrastromal xenotransplantation in rhesus monkeys: A preliminary study. Curr Mol Med. 2018;18:612-21.
- [CrossRef] [PubMed] [Google Scholar]
- Glycerol-preserved corneal tissue in emergency corneal transplantation: An alternative for fresh corneal tissue in COVID-19 crisis. Indian J Ophthalmol. 2020;68:1412-6.
- [CrossRef] [PubMed] [Google Scholar]
- Review of gamma-irradiated sterile cornea: Properties, indications, and new directions. Eye Contact Lens. 2021;47:157-62.
- [CrossRef] [PubMed] [Google Scholar]






