Translate this page into:
A simple method (Schirmer IV) to stimulate reflex tear volume secretion by application of gel ice pack on the closed eyelid in dry eye patients

*Corresponding author: R Balamurugan, Department of Ophthalmology, All India Institute of Medical Sciences, Guntur, Andhra Pradesh, India. bala16690@yahoo.co.in
-
Received: ,
Accepted: ,
How to cite this article: Balamurugan R, Sarma P, Nicodemus DS, Marimuthu Y, Rakesh U. A simple method (Schirmer IV) to stimulate reflex tear volume secretion by application of gel ice pack on the closed eyelid in dry eye patients. IHOPE J Ophthalmol. 2024;3:45-51. doi: 10.25259/IHOPEJO_11_2024
Abstract
Objectives:
We aimed to demonstrate the effectiveness of gel ice pack application on closed eyelids to stimulate reflex tear volume secretion in dry eye patients.
Materials and Methods:
It is a randomized and controlled study. Sixty patients with dry eyes were randomized into two groups of 30 each: Group A (gel ice pack) and Group B (control group, gel pack at room temperature). Tear secretions using a Schirmer strip were measured before and after the gel pack applications and compared.
Results:
The study groups (n = 30 in each) were comparable in terms of baseline characteristics such as best-corrected visual acuity (P > 0.05), intraocular pressure (P > 0.05), and baseline tear volume (P > 0.05). In terms of Schirmer’s reading at 5 min, the application of gel ice pack resulted in a significant increase in lacrimation in Group A (25.77 ± 10.76 for right eye [OD] and 26.43 ± 10.67 for left eye [OS]) when compared to Group B (13.43 ± 3.98 for OD and 13.80 ± 4.55 for OS) in the overall patient population (P < 0.001). The pain score (1–5) was slightly higher in the Group A (1, 1, 0–1) when compared to the control group (0, 0, 0–0), which is statistically significant (P < 0.05). No other adverse events were seen in any of the groups.
Conclusion:
The gel ice pack application on closed eyelids to stimulate reflex tear secretion is a simple, safe, inexpensive, non-invasive, and feasible procedure. Hence, the application of a gel pack (Schirmer IV) stimulate the reflex tear volume secretions could be considered as one of the methods to measure the reflex tear volume secretions.
Keywords
Ice pack test
Gel ice pack
Modified Schirmer test
Maximum tear volume secretion
Lacrimal gland function test
Schirmer 4
INTRODUCTION
The aqueous tear is an important dynamic fluid in the eyes responsible for maintaining ocular surface homeostasis. Most tear volume is secreted mainly by the main lacrimal glands from the orbit and also by the accessory lacrimal glands (the Krause and Wolfring glands) in the conjunctiva.[1] Tear (aqueous) volume deficiency may occur due to aging or other lacrimal gland disorders such as Sjögren’s syndrome (primary or secondary), duct obstructions, or medications.[2] The quantitative assessment of tear volume secretion is measured by various methods such as the Schirmer test, the phenol red thread test, and the tear film fluorescein clearance.[3] Historically, various modifications of the Schirmer I test were done to measure the reflex tear volume secretion, like nasal stimulation (Schirmer II) or making the patients look at the sun (Schirmer III).[4] However, these methods are inappropriate to use in routine clinical practice. New methods have been introduced to induce reflex tear volume secretion using equipment like gas esthesiometer (which stimulates the cornea by mechanical, chemical, and cold stimulation)[5] and i-Onion (which produces a puff of 99.9% carbon dioxide [CO2] at the cornea)[6] or exposure to onion lachrymatory factor.[7] The ice pack test is a commonly performed test to demonstrate ocular myasthenia gravis.[8,9] While performing the test for myasthenia gravis, we noted that there was an increased amount of tear secretion from the eyes, which provided the hypothesis that the application of a gel ice pack on the eyelid stimulates the eye and produces reflex tear secretion. Hence, to prove it, we have designed this study to evaluate the efficacy of gel ice pack application (a gel pack at a cold temperature) to stimulate and measure reflex tear production among dry eye patients.
MATERIALS AND METHODS
Study design
This is a randomized and controlled study conducted in a tertiary care center in India. The study adhered to the principles of Helsinki, and an Institutional Review Board (IEC/2022–23/194) was obtained to perform the study. Informed written consent was taken from the participants before enrolling in the study. Primary objectives: To evaluate the impact of a gel ice pack (gel pack at a cold temperature) on reflex tear production in dry eye patients. Measurement of tear production: Tear production was measured by standard Schirmer’s strips made up of Whatman filter paper 41.[10] The strip was placed on the lateral side of the closed eyelid between the bulbar and palpebral conjunctiva. Outcome measurement: Post-test reflex tear volume was measured and compared with respect to the baseline (change from baseline [CFB], i.e., Schirmer’s reading post-test to Schirmer’s reading at baseline) and percentage times increase (PTI) in tear production compared to baseline at 5 min ([post-test reading at 5 min/pre-test reading at 5 min] × 100). Pain caused by the application of a gel ice pack on the eyelids was measured using the Wong-Baker pain scale (1–5).[11] Inclusion criteria: (1) Participant age above 18 years, (2) patients with dry eyes (as per dry eye workshop (DEWS) criteria),[2] and (3) baseline Schirmer test I (without anesthesia) readings ranging from 0 to 25 mm. Exclusion criteria: (1) Conditions with excessive reflex lacrimation (more than 25 mm) and active ocular inflammation such as conjunctivitis, keratitis, uveitis, and dacryoadenitis. (2) History of trauma, chemical injury, or any other cause of duct obstruction. (3) Glaucoma or raised intraocular pressure (IOP). (4) Severe systemic comorbidities such as congestive heart failure, pulmonary disease, renal disease, uncontrolled hypertension, and diabetes. (5) Any other diseases related to the eyelids and face. (6) Patients on medications that influence the tear secretions.
Randomization
Patients were randomized into two groups: Group A (gel pack at a cold temperature/gel ice pack) and Group B (gel pack at room temperature) using computer-generated randomization as shown in Figure 1. Group A (gel ice pack): A gel pack at a cold temperature (kept in the freezer at −20° and at the ice state) was applied to the closed eyelid for 3 min. Group B (control group): A gel pack at room temperature was applied to the closed eyelid for 3 min. The group allocation order was concealed in a sealed, opaque envelope. Technique for Gel Pack Application (Cold or Normal): We used a gel pack, which is a square-shaped, 8 cm-by-8 cm, non-toxic, safe polymer gel wrapped in a plastic sheet. Before application of the gel pack, the patient was made comfortable at the normal ambient room temperature of 22 ± 2°C. The tears from the conjunctival sac were wiped using tissue paper and waited for 3 min to undergo Schirmer test I (without anesthesia) for basal tear secretion, which was measured using a standard Schirmer strip for 5 min. The gel pack (cold or room temperature) was applied for 3 min on the closed eyelids without touching the margins as shown in Figures 2 and 3. The mist on the gel pack (in the case of an ice pack) was wiped every 30 s. To avoid confounding factors, the gel pack at room temperature was also wiped every 30 s. The ice pack was taken off the eyelid for a few seconds if the patient felt discomfort or pain and reapplied. After 3 min of gel pack application, the Schirmer test was repeated using a Schirmer strip for 5 min. The investigator measuring outcome evaluation is different from the investigator who applied gel packs to the participants. The outcome evaluation done by the investigator is masked to the gel pack allocations (cold or room temperature).
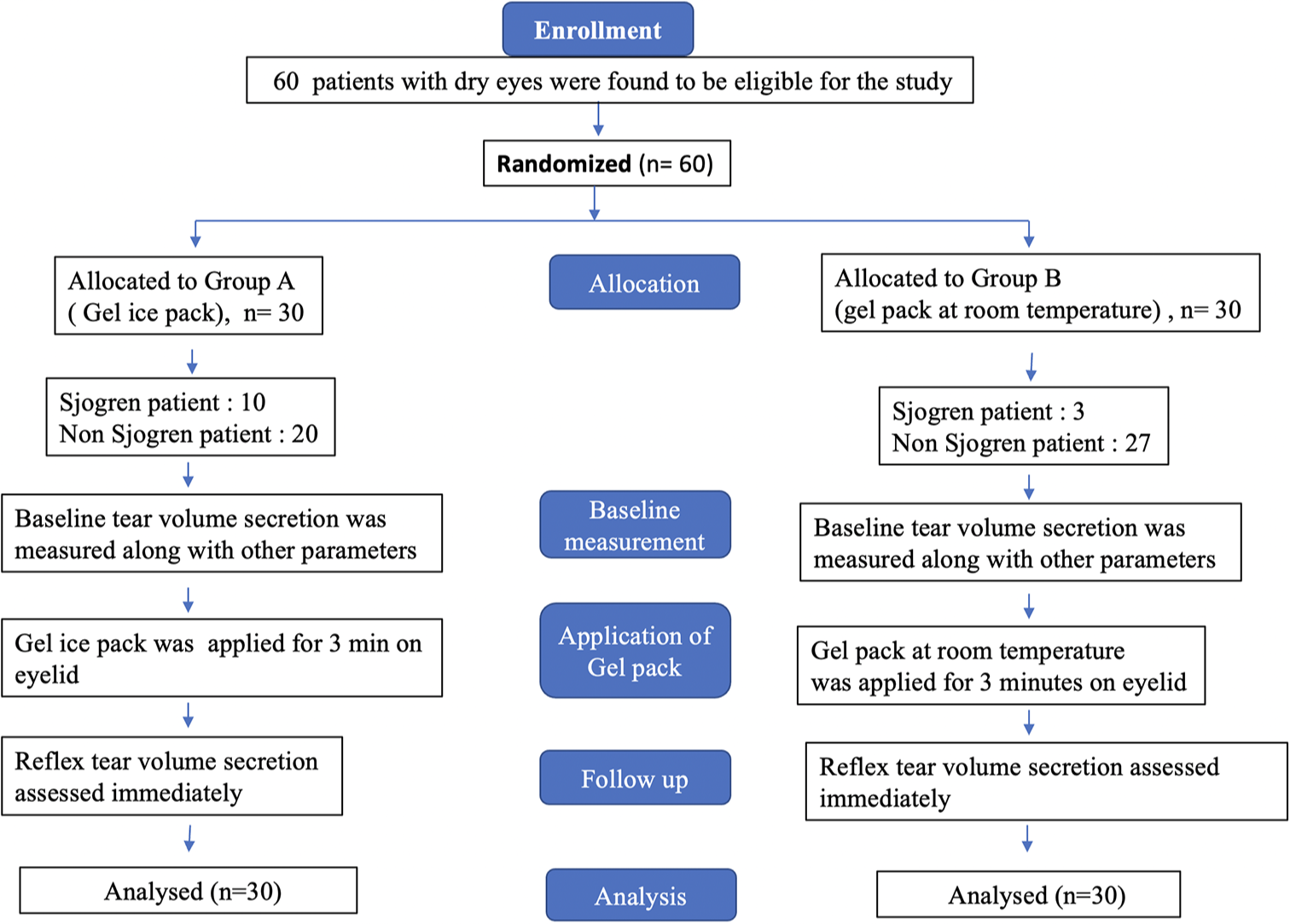
- Participants flow chart.
Statistical analysis
Data were entered into Excel and analyzed using the Statistical Package for the Social Sciences software. Qualitative variables were presented as proportions of the total sample size in that group. Quantitative variables were presented as mean ± standard deviation (in case of normal distribution), or median, inter-quartile range in case data were found not to follow a normal distribution. Hypothesis testing was done using suitable statistical tests as applicable depending on the nature of the variables (qualitative or quantitative). The relationship between two qualitative variables was evaluated using either Chi-square or Fischer exact test (in case the expected value in any of the parameters was <5 in the two-by-two table). In the case of evaluation of the relation between qualitative and quantitative variables, a suitable test was applied depending on the categories of the qualitative variable (in our case, the number of groups = 2) and the distribution of the quantitative variable (normally distributed or not). In the case of a normally distributed quantitative variable, an independent t-test was applied, and if not found to be normally distributed, we used the Mann– Whitney U-test for hypothesis testing. In the case of repeated measurement data, as in all cases, the data were not following normal distribution, so Friedman’s two-way analysis of variance was used for the analysis of data. P < 0.05 was taken as the threshold for statistical significance.
RESULTS
Participant enrolment
The study included a total of 30 patients with dry eyes in each group (Group A, gel ice pack, and Group B, gel pack at room temperature). In Group A, among 30 patients with dry eye diseases, 10 patients were found to be Sjögren’s patients (as per the criteria based on American–European Consensus Group)[2] with aqueous deficiency (i.e., a Schirmer’s reading of <5 mm for 5 min), and 20 were found to be non-Sjögren’s patient. In Group B, among 30 patients with dry eye diseases, three patients were found to be Sjögren’s patients (as per the criteria based on American–European Consensus Group) with aqueous deficiency (i.e., Schirmer’s reading of <5 mm for 5 min), and 27 patients were found to be non-Sjögren’s patients. The details of the screening and participant enrolment are shown in Figure 1.
Baseline characteristics
There was no significant difference in age between the two groups. Most participants in both groups were female (63% and 67%, respectively). Both groups were comparable in terms of age, sex, best-corrected visual acuity (BCVA), IOP, and Schirmer’s reading at baseline. The non-Sjögren patients, as well the Sjögren’s subgroups, were also comparable in terms of baseline BCVA and IOP [Table 1].
| Baseline parameter | Group A (n=30) | Group B (n=30) | P-value |
|---|---|---|---|
| Age (mean±SD) | 42.47±9.864 | 42.07±10.082 | 0.938 |
| Sex (M/F) | 11/19 | 10/20 | 0.787 |
| Pre-test BCVA OD (Snellen in decimal units) (mean±SD) | 0.924±0.1845 | 0.972±0.1086 | 0.229 |
| Pre-test BCVA OS (Snellen in decimal units) (mean±SD) | 0.940±0.2032 | 0.938±0.1677 | 0.978 |
| Pre-test IOP OD (mmHg) (mean±SD) | 14.67±3.133 | 15.73±3.342 | 0.207 |
| Pre-test IOP OS (mmHg) (mean±SD) | 14.60±2.762 | 15.47±3.481 | 0.290 |
| Pre-test Schirmer’s reading at 5 min (OD) (median, IQR, min-max) | 11, 10, 0–23 | 12, 5, 4–20 | 0.250 |
| Pre-test Schirmer’s reading at 5 min (OS) (median, IQR, min-max) | 10, 13, 0–22 | 13.5, 5, 4–21 | 0.180 |
| Non-Sjögren’s Patients (Group A: n=20, Group B: n=27) | |||
| Age (mean±SD) | 39.3±9.331 | 41.7±10.553 | 0.422 |
| Sex (M/F) | 11/9 | 10/17 | 0.221 |
| Pre-test BCVA OD (Snellen in decimal units) (mean±SD) | 0.9830±0.07603 | 0.9689±0.11426 | 0.633 |
| Pre-test BCVA OS (Snellen in decimal units) (mean±SD) | 1.00 | 0.9315±0.17578 | 0.889 |
| Pre-test IOP OD (mmHg in NCT) (mean±SD) | 15.15±3.150 | 15.81±3.442 | 0.501 |
| Pre-test IOP OS (mmHg in NCT) (mean±SD) | 15.05±3.034 | 15.15±3.336 | 0.918 |
| Sjögren’s Patients (Group A: n=10, Group B: n=3) | |||
| Age (median, IQR, min-max) | 49, 11, 37–67 | 47, 42–47 | 0.573 |
| Sex (M/F) | 0/10 | 0/3 | – |
| Pre-test BCVA of the aqueous deficient eye (Snellen in decimal units) (median, IQR, min-max) | 1.00, 0.38, 0.25–1 | 1.00 | 0.371 |
| Pre-test IOP of the affected eye (mmHg in NCT) (median, IQR, min-max) | 14, 3.75, 9–19 | 14, 13–18 | 0.811 |
M: Male, F: Female, BCVA: Best corrected visual acuity, IQR: Interquartile range, IOP: Intraocular pressure, NCT: Non-contact tonometry, OD: Right eye, OS: Left eye, SD: Standard deviation, Group A: Gel ice pack, Group B: Gel pack at room temperature
Impact of gel ice pack application on tear secretion
Schirmer’s reading at 5 min
Application of gel ice pack [Figures 2a and b, 3a and b] resulted in an increase in lacrimation in Group A (P < 0.001) when compared to Group B in both eyes (Right eye [OD] and left eye [OS]) in the overall patient population. Tear hyper-secretion was evident in Group A among both subgroups: Non-Sjögren’s [Figure 2c and d] and aqueous-deficient affected eyes in Sjögren’s patient [Figure 3c and d]. Data are shown in Table 2.
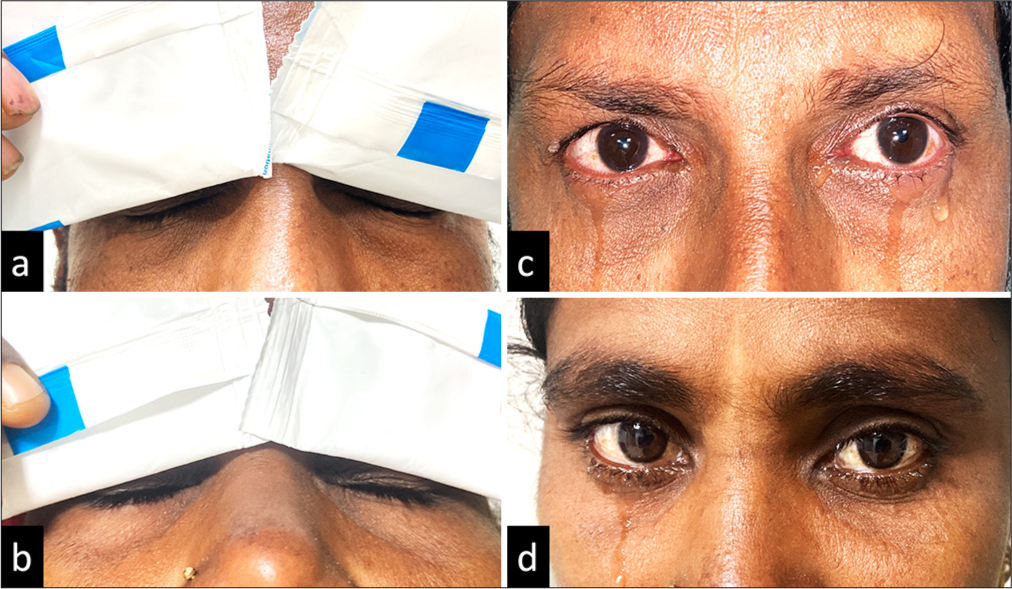
- (a-d) (a and b) The application of gel ice packs at the closed eyelid in non-Sjögren’s dry eye patients. (c and d) The reflex lacrimation induced by the gel ice pack application, which was measured immediately using standard Schirmer strip.
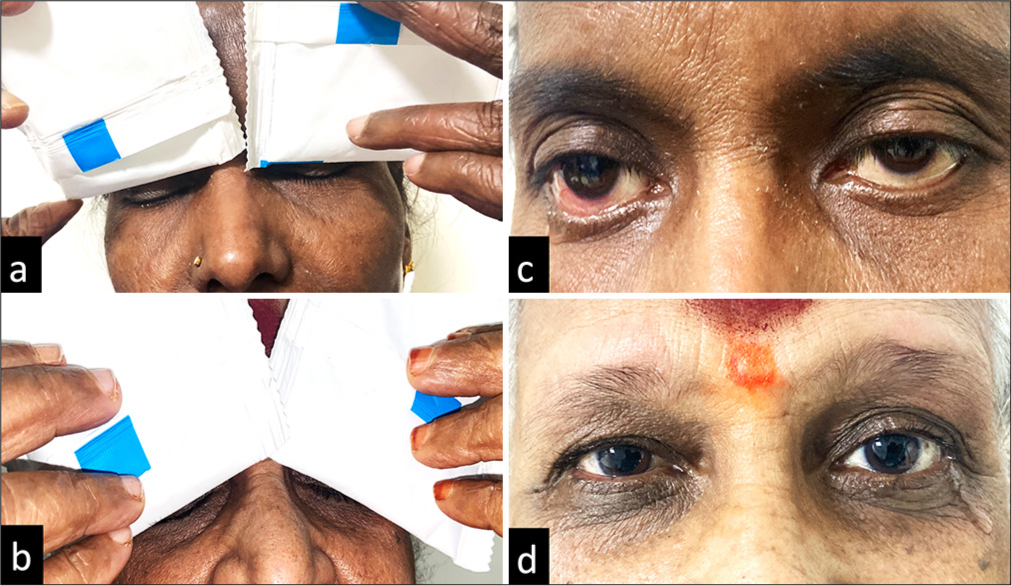
- (a-d) (a and b) The application of gel ice packs at the closed eyelid in aqueous deficient Sjögren’s patients. (c and d) The reflex lacrimation induced by the gel ice pack application, which was measured immediately using standard Schirmer strip.
| Patient population | Parameter | Group A | Group B | P-value (intergroup) | P-value (overall) |
|---|---|---|---|---|---|
| Overall patient population | Schirmer’s reading at 5 min (OD) (mean±SD) | 25.77±10.76 | 13.43±3.980 | <0.001 | N/A |
| Schirmer’s reading at 5 min (OS) (mean±SD) | 26.43±10.676 | 13.80±4.552 | <0.001 | N/A | |
| Non-Sjögren’s patients (OD) | Pre-test Schirmer’s reading at 5 min (median, IQR, min-max) | 14, 5.5, 3–23. MR: 1.92 |
12.5, 3, 10–20. MR: 1.68 |
1.00 | <0.001 |
| Post-test Schirmer’s reading at 5 min (median, IQR, min-max) | 35, 6.25, 13–35. MR: 3.85 |
14, 3.5, 9–21. MR: 2.55 |
0.009 | – | |
| P-value (pre vs. post) | <0.001 | 0.193 | – | – | |
| Non-Sjögren’s patients (OS) | Pre-test Schirmer’s reading at 5 min (median, IQR, min-max) | 13.5, 8.75, 2–22. MR: 2.1 |
14, 4.75, 10–21. MR: 1.72 |
1.00 | <0.001 |
| Post-test Schirmer’s reading at 5 min (median, IQR, min-max) | 32.5, 11, 6–35. MR: 3.85 |
15, 4.75, 10–23. MR: 2.3 |
0.001 | – | |
| P-value (pre vs. post) | <0.001 | 0.850 | – | – | |
| Sjögren’s Patients (aqueous-deficient eyes) | Pre-test schirmer’s reading at 5 min (median, IQR, min-max) | 4, 1.5, 0–5 | 4, 4–5 | 1.00 | 0.041 |
| Post-test Schirmer’s reading at 5 min (median, IQR, min-max) | 13, 16.5, 5–35 | 5, 4–6 | 0.206 | – | |
| P-value (pre vs. post) | 0.027 | 0.343 | – | – |
MR: Mean rank, IQR: Interquartile range, OD: Right eye, OS: Left eye, SD: Standard deviation, Group A: Gel ice pack, Group B: Gel pack at room temperature, N/A: Not available
CFB in Schirmer’s reading (post-test–pre-test reading) at 5 min
The gel ice pack application resulted in an increased change in Schirmer’s reading CFB among the overall population (<0.001), non-Sjögren’s patients (<0.001) and Sjögren’s patients (P = 0.014). Data are shown in Table 3.
| Parameter | Group A | Group B | P-value |
|---|---|---|---|
| CFB in Schirmer’s reading (OD) (median, IQR, min-max) | 13, 12.5, 1–32 | 1, 1, −2–2 | <0.001 |
| CFB in Schirmer’s reading (OS) (median, IQR, min-max) | 17, 13.5, 0–32 | 1, 2, −1–4 | <0.001 |
| CFB in Non-Sjögren’s Patients (OD) (median, IQR, min-max) | 19.5, 10, 3–32 | 1, 1, −2–2 | <0.001 |
| CFB in Non-Sjögren’s Patients (OS) (median, IQR, min-max) | 15, 12, 3–31 | 1, 2, −1–4 | <0.001 |
| CFB in Sjögren’s Patients (aqueous-deficient eyes) (median, IQR, min-max) | 10.05, 14.75, 1–32 | 1, 0–1 | 0.014 |
CFB: Change from baseline, IQR: Interquartile range, OD: Right eye, OS: Left eye, Group A: Gel ice pack, Group B: Gel pack at room temperature
PTI in tear production compared to baseline at 5 min ([post-test at 5 min/pre-test at 5 min] × 100)
The gel ice pack application resulted in an increase in percentage times of baseline lacrimation among the overall patient (<0.001), among the non-Sjögren patient (<0.001), and Sjögren’s patient (P = 0.009). Data are shown in Table 4.
| Parameter | Group A | Group B | P-value |
|---|---|---|---|
| PTI in tear production (OD) (median, IQR, min-max) | 250, 112.4, 120–166 | 109.5, 6.31, 86–125 | <0.001 |
| PTI in tear production (OS) (median, IQR, min-max) | 205.8, 602, 100–1300 | 109, 15, 90–128 | <0.001 |
| PTI in Non-Sjögren’s Patients (OD) | 238, 93, 120–1166 | 109, 5.36, 86–116 | <0.001 |
| PTI in Non-Sjögren’s Patients (OS) | 194, 103, 136–1200 | 109, 16, 90–128 | <0.001 |
| PTI in Sjögren’s Patients (aqueous-deficient eyes) | 460, 733, 125–1300 | 120, 100–125 | 0.009 |
PTI: Percentage times increase, IQR: Interquartile range, OD: Right eye, OS: Left eye, Group A: Gel ice pack, Group B: Gel pack at room temperature
Pain score
The pain score was slightly higher in Group A (median score of 1 in Group A vs. score of 0 in Group B). No other adverse event was seen in any of the groups. Data are shown in Table 5.
| Parameter | Group A | Group B | P-value |
|---|---|---|---|
| Pain score (1–5) (median, IQR, min-max) |
1, 1, 0–1 | 0, 0, 0–0 | <0.001 |
IQR: Interquartile range, Group A: Gel ice pack, Group B: Gel pack at room temperature
DISCUSSION
Ocular tear formation has two components.[10,12] One is the basal component, which is produced continuously to maintain the ocular wet surface. Another is the reflex component caused by noxious stimuli to the eye, such as foreign bodies, infections, and inflammation. The neuronal reflex pathway mediates reflex lacrimal tear formation[1,7,10,12], where afferent innervation is carried by the sensory branch of the trigeminal nerve, which supplies the ocular surface, and the efferent pathway is supplied by the parasympathetic nerve originating from the superior salivary nucleus in the pons and the sympathetic nerve from the superior cervical ganglion. Historically, there are some methods of measuring maximum reflex tear secretion[4] by stimulating the nasal mucosa using a hair brush after anesthetizing the cornea (Schirmer II) and making the patient look at the sun (Schirmer III), which are all of historical significance and practically not in use today.
Tsubota[13] described using a cotton tip to stimulate the nasal cavity in different dry eye subgroups. They found significant reflex tear production in patients with keratoconjunctivitis sicca but not in those with Sjögren’s syndrome. Acosta et al.[5] showed that intense corneal cooling using a gas esthesiometer can significantly stimulate reflex tears by activating polymodal nociceptors. In our study, a simple application of a cold gel pack application could have achieved the cooling of the cornea enough to stimulate the reflex tear volume secretion. Merino et al.[6] used the i-Onion, a device that delivers a 99.9% CO2 puff at 200 mL/min for 3 s to the cornea, and found a significant increase in tear production in normal controls but low response in asymptomatic suspected dry eyes and Sjögren’s syndrome. Higashihara et al.[7] used synthesized onion lacrimatory factor to induce reflex lacrimal secretions, and they found a significant increase in tear meniscus radius. Reflex tear measurement using the above methods, such as nasal stimulation, is not practical now, and other techniques using machines, such as gas esthesiometers and i-Onion, are costlier and not available everywhere.
The ice pack test[8,9] (gel packs kept at a freezer temperature of −20°C) is non-invasive, inexpensive, safe, and routinely performed in clinics to aid in the diagnosis of ocular myasthenia gravis symptoms such as ptosis and diplopia. Application of an ice pack test for 2–5 min on the closed eyelid will lead to improvement in the symptoms and signs (ptosis disappearing). The mechanism behind this could be that lower temperatures cause inhibition of the acetylcholinesterase enzyme and hence increase the availability of the Ach neurotransmitter in the neuromuscular junctions.[9] The ice pack application has to be done using ice wrapped in the plastic cover to avoid ice burn. On performing the ice pack test for diagnosing ocular myasthenia gravis, we noted enormous watering of the eye, which forms the basis of the present study. We have used the same ice pack (a gel pack kept at the freezer temperature of −20°C in solid state) as a safe, non-invasive, and cost-effective mode of external ocular stimulus to induce reflex tear secretions, which could be due to its noxious pain stimulus to the eye[5,6] or cooling effect on the cornea.[5] Pain was noted significantly in the ice pack group when compared to the control group, but it was mild in nature and easily tolerated by the patients. Furthermore, the duration was kept at 3 minutes, compared to the 5-minute duration[9] for ocular myasthenia gravis. The polymodal nociceptor is a major corneal receptor responsible for perceiving pain and irritational sensation.[5] These nerve fibers give the impulse signal on noxious stimuli such as injury or inflammation such as keratitis, which, in turn, increases the reflex tear secretions. There were no adverse effects noted after the test except mild pain and discomfort. The ice pack application was withdrawn every 30 s for approx. 2–3 s to wipe the mist formation, which further reduces the discomfort it causes.
In our present study, the PTI in tear volume in the patients with non-Sjögren’s patients was 238 (93) in the range of 120–1166 for the OD and 194 (103) in the range of 136–1200 for the OS, which was approximately 2 times the baseline secretion. Furthermore, all non-Sjögren’s patients have shown improvements in tear volume secretion. The PTI in tear volume for aqueous-deficient eyes in Sjögren’s patients after ice pack application was 460 (733) in the range of 125–1300, which was approximately 4 times the normal baseline values. This shows that the gel ice pack application significantly increased lacrimation production for the aqueous-deficient Sjögren’s patients, and thereby, the actual potential reserve for lacrimation could be estimated. However, the three affected eyes of Sjögren’s patients did not show any improvement compared to the baseline; this could be due to the severely affected lacrimal glands and poor lacrimal secreting potential. Cold compression and ice application on the eyes are the commonly used treatments for dry eyes.[14] Our study has proven it with evidence of a significant increase in lacrimation, which could be one of the reasons for the relief of symptoms.
Advantages
Our technique for inducing reflex tear production is inexpensive, easy to perform, non-invasive, and safe.
Limitation
Some patients have shown profuse lacrimation [Figure 2c and d, 3c and d] after the gel ice pack application and started dripping, which was not completely measured and crossed the standard 35 mm upper limit of the Schirmer strip in <5 min in many patients. Therefore, the actual secretions after the test must be higher than the recorded values.
Although the application of a gel ice pack induces reflex tear secretion by stimulating the ocular surface, we have not measured the corneal temperature changes after the test.
CONCLUSION
Although only a few techniques have been described to induce and measure the reflex lacrimal tear secretion, they are not practical today due to the invasive and cumbersome nasal stimulation of the hairbrush, and the unavailability of costlier equipment like gas esthesiometer makes such tests not feasible everywhere. Gel ice pack application on the eyelid to induce reflex tear secretion is an effective, inexpensive, safe, and promising technique to measure the potential reserve of the lacrimal glands in both aqueous-deficient and normal aqueous secretion dry patients, and we would name this simple method as Schirmer IV.
Ethical approval
The research/study approved by the Institutional Review Board at AIIMS Mangalagiri, number IEC/2022-2023/194, dated 9th September, 2022.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Review: The lacrimal gland and its role in dry eye. J Ophthalmol. 2016;2016:7542929.
- [CrossRef] [PubMed] [Google Scholar]
- The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5:75-92.
- [CrossRef] [PubMed] [Google Scholar]
- Quantitative assessment of tear production: A review of methods and utility in dry eye drug discovery. J Ocul Biol Dis Infor. 2008;1:1-6.
- [CrossRef] [PubMed] [Google Scholar]
- Tear secretion induced by selective stimulation of corneal and conjunctival sensory nerve fibers. Invest Ophthalmol Vis Sci. 2004;45:2333-6.
- [CrossRef] [PubMed] [Google Scholar]
- Maximal tear secretion evoked by controlled stimulation of corneal sensory nerves in healthy individuals and dry eye subjects. Ocul Surf. 2023;27:80-8.
- [CrossRef] [PubMed] [Google Scholar]
- Using synthesized onion lachrymatory factor to measure age-related decreases in reflex-tear secretion and ocular-surface sensation. Jpn J Ophthalmol. 2010;54:215-20.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosing myasthenia gravis with an ice pack. N Engl J Med. 2016;375:e39.
- [CrossRef] [PubMed] [Google Scholar]
- The use of the ice pack test in myasthenia gravis. JRSM Short Rep. 2010;1:14.
- [CrossRef] [PubMed] [Google Scholar]
- A review of the Schirmer test for tear production. Arch Ophthalmol Chic Ill 1960. 1962;67:564-5.
- [CrossRef] [PubMed] [Google Scholar]
- Prospective evaluation of sedation-related adverse events in pediatric patients ventilated for acute respiratory failure. Crit Care Med. 2012;40:1317-23.
- [CrossRef] [PubMed] [Google Scholar]
- Cold-sensitive corneal afferents respond to a variety of ocular stimuli central to tear production: Implications for dry eye disease. Invest Ophthalmol Vis Sci. 2010;51:3969-76.
- [CrossRef] [PubMed] [Google Scholar]
- The importance of the Schirmer test with nasal stimulation. Am J Ophthalmol. 1991;111:106-8.
- [CrossRef] [PubMed] [Google Scholar]
- Use of artificial tears vs cold compresses for the treatment of dry eye. Invest Ophthalmol Vis Sci. 2013;54:6052.
- [Google Scholar]






