Translate this page into:
Direct healthcare cost and barriers to the medical management of primary open-angle glaucoma in healthcare facilities in Yaounde – Cameroon

*Corresponding author: Christelle Domngang, Department of Clinical Sciences, Universite des Montagnes, Bangangte, Cameroon. dockrystlnoche@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Domngang C, Maguib P, Nanfack Ngoune C, Foutse Y, Kagmeni G. Direct healthcare cost and barriers to the medical management of primary open-angle glaucoma in healthcare facilities in Yaounde – Cameroon. IHOPE J Ophthalmol 2023;2:57-62.
Abstract
Objectives:
The objective of this study was to estimate the direct healthcare cost of glaucoma among patients attending two healthcare services in Yaounde, Cameroon.
Materials and Methods:
This investigation was a hospital-based, observational, and cross-sectional study of 122 glaucoma patients on follow-up from January to August 2021. The study was carried out using a pre-tested questionnaire and analyzed sociodemographic and clinical characteristics, monthly income, investigations performed, medications prescribed, and direct healthcare costs. Data were analyzed using the Statistical Package for the Social Sciences version 24.
Results:
A total of 122 glaucoma follow-up patients participated in the study of whom 73 (59.9%) were male (sex ratio M/F = 1.49). Seventy-one (58.19%) participants were above 60 years. The majority (67.4%) had a higher education level. Over 40% of participants reported a monthly income of <228 Euros. Only 38 (31.1%) had health insurance. During the study year, each patient underwent 2.25 ± 0.84 consultations, 2.76 ± 1.40 ocular pressure measurements, 0.73 ± 0.52 visual field examinations, and 0.32 ± 0.2 gonioscopy. Prostaglandin analogs were the most widely prescribed drugs (71%), followed by β-blockers (Carteolol and Timolol) and carbonic anhydrase inhibitors with frequencies of 62% and 15%, respectively. The direct medical cost of the study population was estimated at 468.47 ± 155.34 Euros. The greatest proportion of out-of-pocket expenses (61.13% of medical costs) concerned the purchase of drugs, with an average cost of 284.16 ± 115.25 Euros. Patients treated in the public sector spent an average of 425.78 ± 38.49 Euros, while those treated in the private sector spent 562.22 ± 139.18 Euros during the study year (P = 0.001). However, health coverage and type of glaucoma did not influence the direct cost of treatment. The main obstacles to glaucoma care were insufficient income followed by forgetfulness.
Conclusion:
The present study shows that almost all participants self-funded their glaucoma management. More than 60% of the direct healthcare cost was devoted to anti-glaucoma drugs and the main barrier to optimal glaucoma care was the lack of financial resources. All stakeholders need involvement, especially health policymakers, to prioritize glaucoma management to make it affordable for patients.
Keywords
Primary open-angle glaucoma
Healthcare cost
Medical treatment
Barrier
INTRODUCTION
In Africa, the most common form of glaucoma is primary open-angle glaucoma (POAG), with a prevalence of 4.2% and an average age of 40–80 years.[1] In Cameroon, this prevalence is 5.5%.[2] Impaired visual function and blindness due to this disease are important determinants of quality of life, particularly in the elderly,[3] making glaucoma a major public health problem. The management of glaucoma has a long-term socioeconomic impact worldwide[4] and is expected to become a major cause of healthcare expenditure. The cost of treatment varies from country to country and depends on the severity of glaucoma.[5] In the United States of America, the cost of therapeutic management of glaucoma is estimated at $2.5 billion per year, and direct costs at $1.9 billion.[4] In Scotland in 2015, for example, the annual cost was 534 Euros per patient,[6] while in Benin, the average monthly cost of medical treatment was estimated at between 28.15 and 169.35 Euros, encompassing direct and indirect treatment costs.[7] It is known that an illness with a financial burden >40% of the household income is likely to have a negative impact on the family finances.[8] As the universal health coverage system has not yet been fully implemented in Cameroon to date, this work aimed to estimate the direct annual cost of medical care for POAG to obtain information that could guide public authorities and heads of institutions to improve the care and follow-up of patients with this condition.
MATERIALS AND METHODS
Study design
We conducted a descriptive cross-sectional study over 6 months (from January 11 to June 15, 2021). The study period ranged from January 1, 2015, to December 31, 2021, in two eye care services of Yaounde, namely, the University Hospital Center of Yaounde and the Innel Medical Center of Yaounde, from the public and private sectors, respectively. These two structures, which contain specialized eye care services, are located in Yaounde, the capital of Cameroon (urban area).
Ethical considerations
Ethical clearance was obtained from the Universite des Montagnes (UdM) Institutional Ethics Committee (n°2021/049/ UdM/PR/CEAQ) and administrative authorizations were granted by health structures. The study was carried out following the principles of the Helsinki Declaration.[9]
The selection criteria were as follows: for inclusion criteria, all participants with a confirmed diagnosis of POAG and a 1-year minimum follow-up and having benefited from medical management; exclusion criteria were all untreated patients, patients who had received non-medical treatment for their POAG, and incomplete records.
The sample size was calculated using the Cochran formula with the use of the prevalence of POAG reported by Ellong et al.[2] The minimum study size was 80 participants. A total of 122 glaucoma patients participated in the study.
Outcome variables
Before patient selection, patients’ records were reviewed to ensure eligibility for inclusion as noted above. A pretested questionnaire containing both open- and closed-ended questions was administrated to the participants. The following data were collected: sociodemographic variables (age, gender, educational level, monthly income, health insurance, and city of residence); clinical variables (history, visual acuity [VA], cup/disc (C/D), and intraocular pressure [IOP]); paraclinical variables (visual field, optical coherence tomography, and gonioscopy); classification of the POAG severity according to Hodapp–Parish and Anderson criteria;[10] prescribed medical treatment (number of drug molecules and therapeutic classes); variables relating to the direct health cost (consultation, investigations related to glaucoma, and medications); variables relating to direct non-medical cost (transportation); and barriers to optimal glaucoma care (insufficient financial income, distance, and forgetting to go to the hospital or to take medication).
Statistical analysis
Data storage and analysis were performed using Excel 2013 and Statistical Package for the Social Sciences version 24.0, respectively. Quantitative variables were expressed in mean and stand deviation and categorical variables in frequency and percentages. Quantitative variables were compared using the Student t-test. The threshold of significance was set at P < 0.05.
RESULTS
Sociodemographic characteristics
A total of 122 glaucoma follow-up patients participated in the study of whom 73 (59.9%) were male (sex ratio M/F = 1.49). Seventy-one (58.19%) participants were above 60 years, and the majority (67.4%) had higher education levels. Sociodemographic characteristics are shown in [Table 1].
| Characteristics | Population (n=122) | Frequency (%) |
|---|---|---|
| Age (year) | 59±16 (10–86) | |
| Sex | ||
| Male | 73 | 59.9 |
| Female | 49 | 40.1 |
| Education level | ||
| Tertiary | 82 | 64.4 |
| Secondary | 19 | 15.9 |
| Primary | 8 | 6.8 |
| None | 13 | 9.9 |
| Residency | ||
| Yaounde | 100 | 81.9 |
| Other | 22 | 18.1 |
| Health insurance | ||
| Yes | 38 | 31.1 |
| No | 84 | 68.9 |
| Monthly income [EUROS] | ||
| ≤152.44 | 43 | 31.2 |
| [152.44–228.67] | 11 | 9.00 |
| [228.67–304.89] | 28 | 22.9 |
| [304.89–381.12] | 30 | 24.6 |
| >381.12 | 15 | 12.2 |
n: Number of participants
The predominant salary range was 304.89–381.12 Euros, with a frequency of 24.6%. The majority of participants (81.9%) lived in Yaounde. Only 31% (n = 38) of the study population had health insurance: of these, 34 (27.8%) respondents were treated in the private sector and 4 (3.3%) in the public sector healthcare facilities.
Clinical characteristics and severity stages
The most comorbidities found were arterial hypertension (24.7%; n = 30) followed by type 2 diabetes (5.8%; n = 7). A total of 82 (67.2%) participants had no known comorbidity. Participants with one or more glaucoma-affected relatives represented 3.3% (n = 4) of the study population.
[Table 2] shows the clinical and paraclinical characteristics of the study population.
| Characteristics | Right eye | Left eye |
|---|---|---|
| Mean±SD (Min–Max) | Mean±SD (Min–Max) | |
| Visual acuity (LogMAR) | 0.52±0.13 (0–2.6] | 0.65±0.17 (0–2.6) |
| Intraocular pressure (mmHg) | 23.5±6.6 (10–48] | 23.5±8.6 (9–60) |
| Cup/disc | 0.73±0.2 (0.2–1) | 0.79±0.2 (0.2–1) |
| Visual field (MD) | 1.9±6.6 ([−29]; −40) | 13.6±6.2 ([−4]; −40) |
| Glaucoma severity | n | n |
| Severe glaucoma MD: >−12dB |
63 | 64 |
| Moderate glaucoma MD: <−12dB |
1 | 0 |
| Low glaucoma MD: <−6dB |
58 | 58 |
MD: Mean deviation, dB: decibel, SD: Standard deviation, n: Number of eyes
The mean VA of the study population ranged from 0.52 (decimal value = 3/10) to 0.65 (decimal value = 2/10), that is, borderline VA according to the LogMAR scale. A high IOP, with a mean value of 23.5 mm Hg, was found. The cup/disc ratio was abnormal, ranging from 0.8 to 0.9. A large proportion of participants had an impaired visual field, with right and left eye values of 11.9 and 13.6, respectively. In the present series, more than half of the studied patients (52%) had severe glaucoma.
Regarding medical treatment, 60.7% (n = 74), 31.9% (n = 39), and 7.4% (n = 9) of participants were on monotherapy, bitherapy, and tritherapy, respectively. The combination of three drugs was only prescribed for patients with severe glaucoma.
Prostaglandin analogs (Travoprost and Latanoprost) were the most widely prescribed drugs, with a frequency of 71%, followed by β-blockers (Carteolol and Timolol) and carbonic anhydrase inhibitors with frequencies of 62% and 15%, respectively. The α-2 adrenergic antagonists were the least used drugs (2%) [Figure 1].
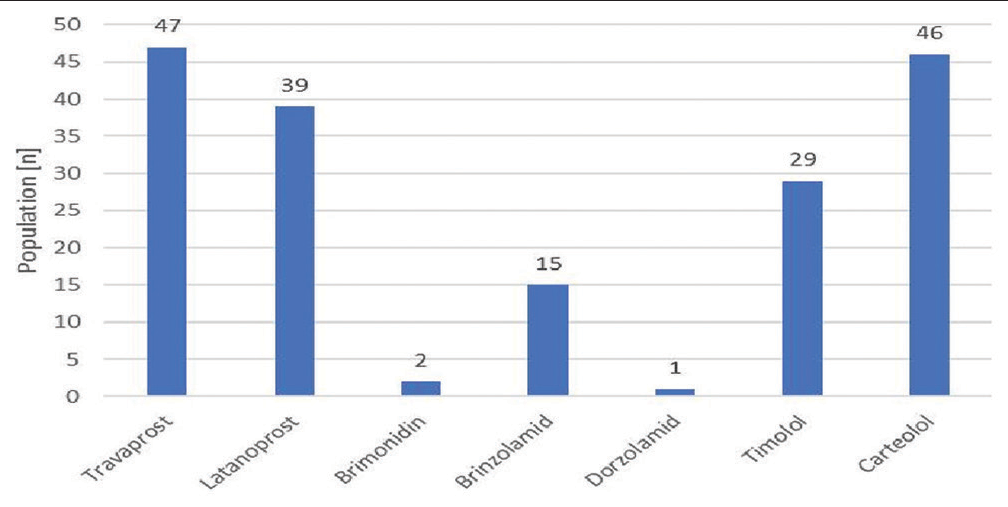
- Distribution of ocular antihypertensives used by the study population.
Evaluation of direct costs
Analysis of investigations shows that 275 consultations, 337 ocular pressure measurements, 195 fundus examinations, 90 visual fields, and 39 gonioscopy procedures were carried out during the year of the study. [Table 3] shows the average number of medical procedures performed on the study population, as well as the average cost of each procedure performed during the year.
| Variables | Number of medical investigations Mean±SD | Min–Max |
|---|---|---|
| Consultation | 2.25±0.84 | 1–4 |
| Intraocular pressure | 2.76±1.40 | 0–9 |
| Fundus examination | 1.59±0.80 | 1–4 |
| Visual field | 0.73±0.52 | 1–3 |
| Gonioscopy | 0.32±0.20 | 0–2 |
| Variables | Cost (Euros) Mean±SD | Min–Max |
| Consultation | 35.67±10.36 | 7.62±68.60 |
| Tonometry | 31.70±10.06 | 2.28±64.02 |
| Fundus examination | 26.06±11.12 | 3.81±91.46 |
| Visual field | 34.75±16.77 | 19.05±91.46 |
| Gonioscopy | 19.81±11.73 | 6.86±30.49 |
| Optical coherence tomography | 32.47±7.16 | 27.44±45.73 |
| Drugs | 284.16±115.25 | 113.42±552.47 |
| Medical direct cost | 464.81±155.65 | 186.59±837.55 |
| Non-medical direct cost | 4.57±2.74 | 0.30±60.97 |
| Total direct cost | 468.47±155.34 | 187.51±845.17 |
SD: Standard deviation
The annual direct medical cost for the study population was estimated at 468.47 ± 155.34 Euros, with extremes of 187.51 and 845.17 Euros during the study year. The greatest proportion of out-of-pocket expenses (61.13% of medical costs) concerned the purchase of drugs, for an average cost of 284.16 ± 115.25 Euros, with extremes of 113.42 and 552.47 Euros.
[Table 4] shows the annual direct cost according to certain sociodemographic characteristics.
| Variables | Mean±SD (Euros) | P-value |
|---|---|---|
| Direct cost according to sociodemographic profile | ||
| Health-care facilities | ||
| Public sector | 425.78±38.49 | 0.001 |
| Private sector | 562.22±139.18 | |
| Residency town | ||
| Yaounde | 462.83±147.87 | 0.61 |
| Other | 473.04±54.88 | |
| Health insurance | ||
| Yes | 498.35±154.88 | 0.26 |
| No | 442.10±155.19 | |
| Direct cost according to glaucoma severity | ||
| Severe glaucoma | ||
| Yes | 534.17±150.77 | 0.06 |
| No | 469.23±91.46 | |
| Moderate glaucoma | ||
| Yes | 466.18±108.23 | 0.9 |
| No | 457.80±146.35 | |
| Low glaucoma | ||
| Yes | 434.63±141.77 | 0.1 |
| No | 484.32±159 | |
SD: Standard deviation
Patients treated in the public sector spent an average of 425.78 ± 38.49 Euros, while those treated privately spent 562.22 ± 139.18 Euros during the study year, with a statistically significant difference (P = 0.001). Patients living in Yaounde spent a total of 462.83 ± 147.87 Euros over the year. Patients with private health insurance spent an average of 498.35 ± 154.88 Euros.
Analysis of the barriers to optimal glaucoma care shows that the main barrier was insufficient income as reported by sixty (48.17%) participants [Figure 2].
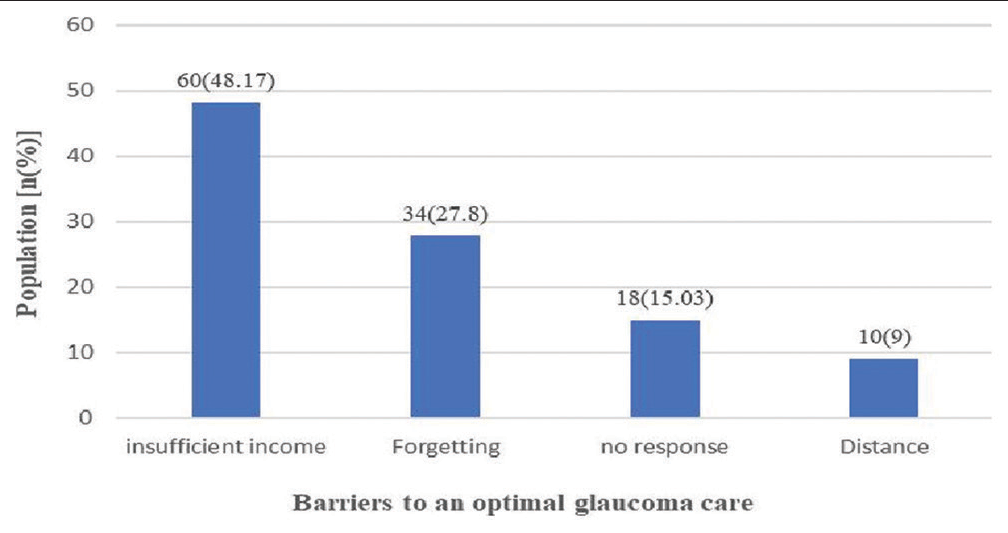
- Distribution of the study population according to barriers to optimal glaucoma management.
DISCUSSION
This study assessed the direct cost of managing POAG and identified obstacles to optimal management. Glaucoma is the 2nd leading cause of blindness after cataracts in Africa, with the primary open-angle form being the most common.[11,12] According to the literature, its prevalence is estimated at 4.16% in sub-Saharan Africa.[11]
In the present study, more than 60% of participants were aged above 60 years. That can explain the proportion of severe glaucoma and comorbidity among the study population. Some systemic risk factors of glaucoma were present in the study population, namely, male gender (59.9%) and arterial hypertension (24.7%). According to the literature, ocular and systemic risk factors for this condition as age, gender, and vascular diseases have been identified in MelanoDerm populations.[13]
In the present study, the severe form of glaucoma was the most frequent (more than 50%) and the average cup-to-disc ratio was 0.8 and 0.9 in the right and left eyes, respectively. Actually, POAG is frequently detected late and leads to blindness in African settings.[2,11] Omgbwa et al. reported bilateral and unilateral blindness rates of 19.7% and 14.1%, respectively, among 1927 cases of blindness.[12] Ellong et al. found a prevalence of bilateral and monocular blindness due to glaucoma of 8% (n = 108) and 32.9% (n = 441), respectively, in glaucoma patients in a sample of 24,462 records collected.[2]
In the present study, 67.7% of participants had a higher level of education. Socioeconomic factors and awareness are key elements involved in the progression of glaucoma and the onset of blindness in MelanoDerm glaucoma patients. Lifelong follow-up is an integral part of glaucoma management, and failure to provide adequate follow-up can have serious consequences for patients.[14]
Participants had performed between 1 and 4 consultations/ year. In addition, an average of two fundus examinations was performed over the year. In terms of explorations, participants performed less than one gonioscopic examination and less than one visual field examination during the year. The gonioscopic examination was not performed systematically. Examination of the optic nerve head is an important element in the diagnosis of glaucoma. According to Kyari et al., in a study of 250 ophthalmologists in Nigeria, optic disc examination in favor of glaucoma was the trigger for glaucoma management in 85% of cases. Moreover, a gonioscopic examination was performed in only 56% of cases.[15]
The yearly average direct cost of medical care was 468.47 ± 155.34 Euros, equivalent to a monthly budget of 39.04 ± 12.94 Euros. The global direct cost reported in the present study was higher compared to that reported by Vonor et al. (277.69 ± 132.42 Euros).[16] The average direct cost of glaucoma care was statistically higher in the private sector than in the public sector (P = 0.001). However, health coverage and type of glaucoma did not influence the direct cost of treatment. Medical treatment accounted for the highest proportion of the total direct cost of glaucoma, at over 60%. Monotherapy was the most common form of treatment, with prostaglandin agonists the most widely used molecules. However, prostaglandin agonists, which are increasingly preferred in the medical treatment of glaucoma for their efficacy, are more expensive than beta-blockers. In the present study, the monthly cost of medical treatment was 23.68 ± 9.6 Euros. This cost is above the average monthly costs reported by Sounouvou et al., in Benin[7] and Bello, in Nigeria[17] which were 17.8 ± 10.8 Euros and 21.3 Dollars (= 19.74 Euros), respectively. This could explain the difficulty of adherence to medical treatment in Africa[18,19] and more particularly in Cameroon. Over 40% of participants in the present study reported a monthly income of <228 Euros and 68.8% had no health insurance or universal health coverage. Thus, the economic burden of glaucoma care is on the individual or his/her relatives. Our results are superimposed on those of Sounouvou et al. in Benin, who found that financial responsibility was assumed by the patient in 72% of cases due to a lack of health coverage.[7] Universal health coverage is the ideal solution for the management of glaucoma, as it is a lifelong disease. Moreover, as 58.19% of patients are more than 60 years old, they are in the age group associated with more comorbidity that increases the economic burden.[17]
The main obstacle to glaucoma care was insufficient finance, as reported by more than half of the participants. One of the major reasons for poor follow-up in glaucoma management in Africa is financial; this is due to the high cost of care[19] and the lack of financial resources for the patients.[18] The second barrier to appropriate management reported by participants is forgetfulness. Glaucoma management is a chronic pathology with high morbidity requiring regular awareness-raising to improve patient support and, above all, adherence to treatment. As such, it requires the strong involvement of the entire healthcare system in communication, screening, management, and active follow-up of patients.[13] This would help preserve patients’ vision over the long term.
Potential limits of this study include the fact that we did not assess the household income of each participant. Moreover, in the case of source funding, the details of the family support instead of personnel one are not detailed. However, the present study, as the first one on the cost of glaucoma care in Cameroon, highlights the need for the involvement of all stakeholders to better manage glaucoma in our context.
CONCLUSION
The present study shows that the majority of participants finance their glaucoma management. The total direct cost of medical care during the year was 469.16 ± 155.5 Euros, of which 284.58 ± 115.4 Euros were devoted to anti-glaucoma drugs. The main barrier to optimal glaucoma care was the lack of financial resources. Cameroonians cannot afford to self-fund their glaucoma care in the long term. The introduction of universal health coverage for all and the strengthening of health systems would contribute to improving the management of this disease.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of Artificial Intelligence (AI)-Assisted Technology for assisting in the writing or editing of the manuscript and no images were manipulated using the AI.
Financial support and sponsorship
Nil.
References
- Evaluation of quality of life in patients with primary open-angle glaucoma in an urban setting. Mali Méd (En ligne). 2019;34:34-8.
- [Google Scholar]
- Direct costs of glaucoma and severity of the disease: A multinational long term study of resource utilisation in Europe. Br J Ophthalmol. 2005;89:1245-9.
- [CrossRef] [PubMed] [Google Scholar]
- Cost of glaucoma treatment in a developing country over a 5-year period. Medicine (Baltimore). 2016;95:e5341.
- [CrossRef] [PubMed] [Google Scholar]
- Direct healthcare costs of glaucoma treatment. Br J Ophthalmol. 2013;97:720-4.
- [CrossRef] [PubMed] [Google Scholar]
- Socioeconomic aspects of the management of primary open angle glaucoma in Benin. J Fr Ophtalmol. 2015;38:809-14.
- [CrossRef] [PubMed] [Google Scholar]
- Exploring dynamics in catastrophic health care expenditure in Nigeria. Health Econ Rev. 2022;12:22.
- [CrossRef] [PubMed] [Google Scholar]
- Declaration of Helsinki-Ethical principle for medical research involving human subjects. 2018. Paris: World Medical Association; Available from: https://www.wma.net
- [Google Scholar]
- Epidemiology of glaucoma in Sub-Saharan Africa: Prevalence, incidence and risk factors. Middle East Afr J Ophthalmol. 2013;20:111-25.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and causes of blindness at a tertiary hospital in Douala, Cameroon. Clin Ophthalmol. 2011;5:1325-31.
- [CrossRef] [PubMed] [Google Scholar]
- The current status of glaucoma and glaucoma care in Sub-Saharan Africa. Asia-Pac J Ophthalmol. 2018;7:375-86.
- [Google Scholar]
- The capacity of eye care services for patients with glaucoma in Botswana. Ophthalmic Epidemiol. 2015;22:403-8.
- [CrossRef] [PubMed] [Google Scholar]
- Ophthalmologists' practice patterns and challenges in achieving optimal management for glaucoma in Nigeria: Results from a nationwide survey. BMJ Open. 2016;6:e012230.
- [CrossRef] [PubMed] [Google Scholar]
- Direct cost of primary open angle glaucoma management. Open J Ophthalmol. 2022;12:352-61.
- [CrossRef] [Google Scholar]
- Economic burden of glaucoma in Nigeria. Estimating the direct health care cost in a tertiary clinic. Niger J Ophthalmol. 2023;31:25-31.
- [Google Scholar]
- Follow-up and adherence to glaucoma care by newly diagnosed glaucoma patients in Enugu, Nigeria. Ophthalmic Epidemiol. 2019;26:140-6.
- [CrossRef] [PubMed] [Google Scholar]
- So let me find my way, whatever it will cost me, rather than leaving myself in darkness: Experiences of glaucoma in Nigeria. Glob Health Act. 2016;9:31886.
- [CrossRef] [PubMed] [Google Scholar]







