Translate this page into:
Surveillance and guidelines update process at NICE

*Corresponding author: Kriti Shukla, Indian Health Outcomes, Public Health and Economics Research Centre, Indian Institute of Public Health, Hyderabad, Telangana, India. kriti.shukla@iiphh.org
-
Received: ,
Accepted: ,
How to cite this article: Shukla K, McGuire H, Dominguez PP. Surveillance and guidelines update process at NICE. IHOPE J Ophthalmol 2022;1:53-5.
Abstract
The discoveries, new body of evidence, interventions, and new knowledge are inherent to science. Therefore, guidelines and recommendations also need to be under constant surveillance to update and embrace the new evidence, resulting in improved quality and safety. This article covers the process and decision-making process around the guidelines. The article captures the essence of discussion in the knowledge exchange seminar series between national institute for health and care excellence (NICE) and IHOPE, which was organized as a part of the collaborative project between NICE International and IHOPE to develop and implement evidence-based clinical guidelines in India. This paper is the fourth in the series, and further information on the other papers can be found at this link.
Keywords
Surveillance
Guideline development process
Guideline update process
Medical science and scientific knowledge are dynamic and ever-evolving. Diagnostic tools, technologies, interventions are constantly being developed and, and the evidence to support their use is also being generated continuously. This calls for regular updates of guidelines and recommendations as well. However, issues related to the right timeline to use, which surveillance and update processes to use, and challenges to keeping up to date with evidence are critical questions that come up during the lifespan of a guideline. The session aimed to understand the decision-making process around which guidelines to update and how to update them.
PRINCIPLES AND PROCESS OF THE GUIDELINE UPDATE
One of the core principles of guideline production (paper 1 developing and quality-assuring guidelines in this series) is to undertake regular reviews of guidelines and content. [Figure 1] represents an overview of the process and steps involved in developing guidelines at the national institute for health and care excellence (NICE). Surveillance is listed as a looping step through which new evidence can be identified that could trigger a review and potential update of a guideline as part of a 5-year standard review cycle.
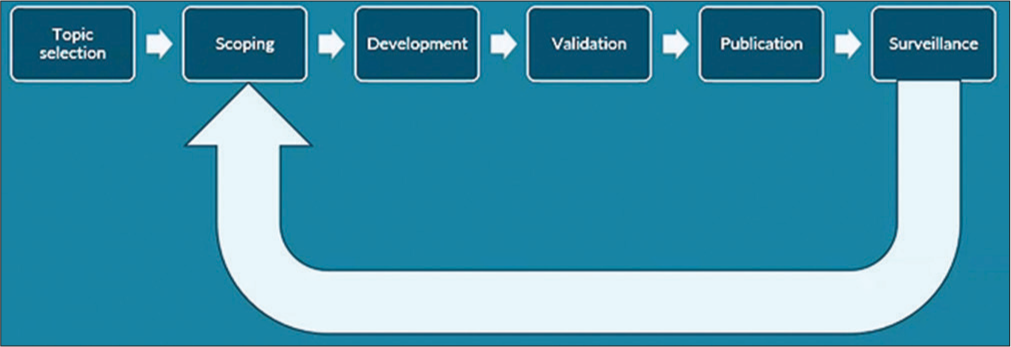
- Overview of the guideline surveillance and updating process (taken from NICE International and IHOPE session 4 - Surveillance and guidelines updating process. January 4, 2022).
NICE has also retained the ability to conduct exceptional reviews. The triggers for these reviews include consideration of external inquiries received by NICE and feedback from stakeholders, the guideline developing team, or the guideline committee members. When deciding on whether to proceed with an update or not, NICE also considers the impact that a change to recommendations in the guideline in question may have on related guidelines. For instance, if the changes in one guideline would lead to changes in a related guideline, then an exceptional review of the impacted guideline would be warranted. This process is outlined in [Figure 2].

- Detail of the standard surveillance review process followed at NICE (taken from NICE International and IHOPE session 4 - Surveillance and guidelines updating process. January 4, 2022).
Understanding why an existing recommendation was made (e.g., as outlined in the rationale and impact section or the committee discussion of the evidence section of a guideline) (paper 1 –developing and quality-assuring guidelines) allows the surveillance team to determine if new evidence may impact a current recommendation. For example, there may be a disconnect between recommendations based on committee consensus rather than evidence alone and a surveillance program that relies on new evidence. This highlights the importance of the rationale and impact and committee discussion sections to outline the contextual underpinning of the recommendations.
There may also be conflicting expert opinions about whether to update a guideline or not. So NICE consults with stakeholders on this decision. Each decision about updating a particular guideline or not is then published on the NICE website. NICE currently has over 350 guidelines and over 20,000 recommendations that are based on evidence, consensus, or a mixture of both. Prioritizing which guideline or group of guidelines should be updated, not updated, or stood down is essential.
The “living guideline” approach that NICE is adopting leads to decisions on how and when new evidence should be included. Some topics may have a more rapidly changing evidence base than others. The process to decide how these guidelines should be updated will need to be different. Another consideration of the “living guideline” approach is whether new evidence may support the existing recommendation. In this scenario, an immediate update may not be required. However, this decision should be communicated to the end-users.
Being clear with end-users about the status of the underpinning review and when the recommendations may be updated is important. Collaboration between guideline developers on surveillance and updates is crucial to maximize efforts and minimize duplication. When updating guideline recommendations, a balance should be achieved so health-care professionals can be kept up to date but not overburdened by frequent marginal updates.
DISCUSSION
Challenges in surveillance and the update of guidelines are common across jurisdictions, including India and the UK. Balancing the increasing evidence base with contextual change, for example, new national policies or new interventions is paramount. The fact that each recommendation may be based on different types of evidence and may overlap across guidelines makes surveillance and updating an enormous task. Partnerships with other organizations are essential to achieve a common objective and reduce duplication of efforts, thus allowing resources to be re-directed to other priority areas. A “living guideline” approach may also be useful in managing high workload levels.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.






