Translate this page into:
Impactful clinical trial and efficient clinical research

*Corresponding author: Yogita Prabhakar Kadam, Clinical Optometrist and Research Associate, eyeSmart EMR, IHOPE, L.V. Prasad Eye Institute, Hyderabad, Telangana, India. yogi.star910@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kadam YP. Impactful clinical trial and efficient clinical research. IHOPE J Ophthalmol. 2024;3:27-30. doi: 10.25259/IHOPEJO_26_2023
INTRODUCTION
Clinical trials are rigorous scientific investigations designed to evaluate the safety and effectiveness of investigational products, including medicines, vaccines, and devices. Rigor is reflected in the careful planning, ethical considerations, and meticulous methodologies employed throughout the lifecycle of the trial. From study design to participant recruitment, data collection, and analysis, the emphasis on quality ensures that the results are reliable, reproducible, and ethically sound.[1-3] Designing a clinical trial with high impact involves careful consideration of various factors, including research questions, outcomes, participant selection, and statistical methodologies [Figure 1].[4] Successful completion of clinical trials depends on the retention of the participants enrolled.[5]
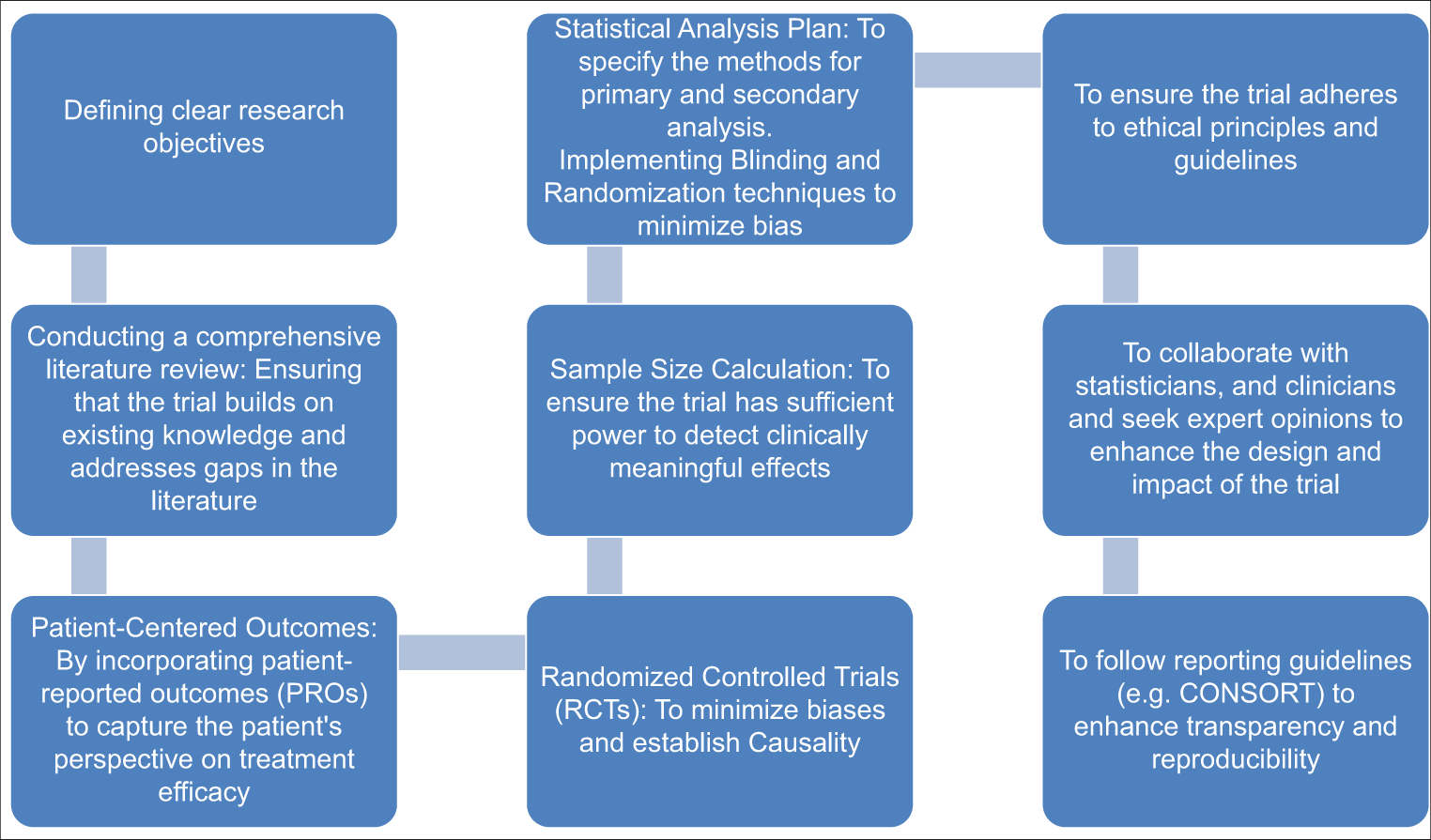
- Process of an impactful clinical trial
GUIDELINES FOR CLINICAL TRIALS
METHODOLOGIES FOR CLINICAL TRIALS
International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use International Council for Harmonisation (ICH) Harmonized Tripartite Guideline: Statistical Principles for Clinical Trials
Sample Sizes for Clinical Trials with Normal Data
Statistical Principles or Clinical Trials: Updated ICH E9 Guideline.
SUPPORT NEEDED BY TRIALISTS TO CONDUCT TRIALS AT THE ORGANIZATION
To conduct successful clinical trials within an organization, many support systems are important, such as
CHALLENGES FACED WHILE DOING LARGE MULTICENTER COLLABORATIVE TRIALS IN INDIA
Executing large multicenter collaborative trials in India poses challenges rooted in the country’s diverse healthcare landscape. Regulating complexities, such as navigating the approval process from the different ethical committees and regulatory bodies, demands meticulous planning and coordination.[10] Logistic hurdles, including the transportation of biological samples and ensuring consistent data collection across centers, require strong operational strategies.[11] Lack of consistency in infrastructure and expertise among participating centers necessitates standardized training programs to ensure data quality and protocol adherence. To address these challenges, a collaborative approach is required with strong leadership and adherence to international ethical and methodological standards.
The Coronavirus Disease 2019 (COVID-19) pandemic has emphasized the need to reimagine and streamline the drug development process, especially for multicountry multicentric clinical trials. The prolonged timelines and escalating costs have prompted a critical reassessment. The importance of moving and acting quickly in a particular trial design, leveraging real-world evidence, and enhancing global collaboration is very important.
Initiating a clinical study involves navigating the complexities of ethics and regulations. India became a popular place for many studies, especially during COVID-19, due to its decision-making quickly. Investigational drug trials strictly adhere to protocol and procedures, but the pandemic brought in the idea of being more flexible, called “Adaptive design.” This means researchers can make planned changes and approve as they learn more, without losing trustworthiness. While an adaptive design increases efficiency, it would need a strong infrastructure. Infrastructure can be in terms of data management and analysis because it is data that helps make a quick decision. Without the necessary infrastructure for data management, data analysis, and real-time decision support, making decisions based on data in a boardroom setting becomes difficult. All organizations cannot achieve the required speed in processing and utilizing data for timely decision-making. While adaptive design trials are flexible and easier to execute, they have certain limitations in terms of infrastructure and expected outcomes. Hence, efficiency in clinical research is ensured by asking the right questions and choosing the right design. Efficiency translates to the money and time invested in conducting quality research.
Electronic health records are one way of improving efficiency. Data in health care are extracted from multiple sources, and it is directly connected to healthcare delivery[12], wherein big data can be recorded quickly and efficiently ensuring reproducibility.
In the realm of research, securing funding is highly important. Grant proposals reflect the ambitious scope of what researchers aim to achieve; however, there exists a struggle between the envisioned scope of work and the financial constraints of the allocated funds. An illustrative case is the L.V. Prasad Eye Institute, which reportedly allocates 12% of its budget to research – a commendable commitment to research leading to advancements in health care.
Research funding is always limited but highly impactful. At the Indian Council of Medical Research (ICMR), the funding is divided into small grants, intermediate grants, and larger grants for advanced research depending on the needs and the size of the project. The ultimate goal of any research is not the publication but one that advocates a policy or a practice change. It may not be a groundbreaking or a huge impact as these changes always come in increments. A thoughtfully designed and reviewed research proposal ensures these incremental changes impact patient management in whatever way possible. Research funding should be able to meet the requirements of the research with optimal utilization of resources.
NAVIGATING THROUGH CHALLENGES IN CLINICAL TRIALS
Conducting clinical trials faced unprecedented challenges during the COVID-19 pandemic, especially for trials requiring in-person interactions. Initiatives such as home care emerged as a response to these challenges and ensured compliance with the investigational drug and the study procedures and visits. This prompted a broader discussion about the possibility of decentralizing clinical trials. Recognizing the problems faced by different studies, a hybrid model is being envisioned. While decentralization of trials is feasible for observational and epidemiological studies, it poses challenges for more intensive and specialized trials like trials in oncology. Striking a balance between centralization and decentralization ensures the integrity of data, adherence to ethical standards, and patient safety.
IMPORTANCE OF PARTICIPANT RETENTION IN RESEARCH
Ensuring quality research extends beyond sponsor or investigator interests. Patient participation is a vital aspect. A robust network involving patients, their families, and hospital staff amplifies the study’s reach. In India, where emotional connections play a significant role, word-of-mouth references from participants are invaluable. Retaining participants requires a patient-centric approach. A few recommended practices, such as
Building rapport
Providing escort services
Maintaining constant communication and involvement of a national study coordinator
Retention camps that further engage participants
Appreciation for the participant’s commitment to their role through mementos at the study’s completion fosters a sense of accomplishment and can contribute to a 99% retention rate. A crucial aspect of patient-centric protocols is ensuring user-friendly processes, compliance with study procedures, and scheduled follow-up visits.
PATIENT CENTRICITY OF CLINICAL TRIAL DESIGN: ELEVATING THE IMPORTANCE OF PROs
In the dynamic landscape of clinical trial design, understanding the patient’s perspective is paramount. The patient’s desired outcomes, their experience with symptoms, and the impact of the disease on their daily life significantly shape the trial’s objectives. While various outcomes, such as clinical efficacy, safety, and economic considerations, are crucial, PROs hold a distinct significance. PROs are a direct reflection of the patient’s experience – capturing changes in symptoms or improvements in their well-being over time. Despite the inherent value of PROs, they are sometimes overshadowed by more clinically focused endpoints. For example, in cardiovascular trials, while clinicians may focus on the success of interventions like stents, PROs often provide a nuanced understanding of the patient’s overall health. Hence, awareness of PROs in a clinical trial is very important for a clinician while conducting the clinical trial. Systematic review is at the top most in the clinical research pyramid. At ICMR, there is a whole wing which is named as Center for Evidence Synthesis and a dedicated team of scientists.
In a clinical trial, it is very important to codesign, coconduct, and codisseminate what we do, and some of the work that we are doing, especially in low- and middle-income countries such as Nigeria and Srilanka involves a lot of formative work. Engagement of communities is done for developing the research question stage, and they are part of the team brainstorming the right question, the research methods, and expected outcomes that might be of interest to the patient, which helps improve efficiency, participant retention, cost-effectiveness of the trials, resulting eventually in impactful clinical trials.
In terms of a systematic review, policymakers should have a methodology. A systematic review is an essential evidence-synthesis tool that creates evidence of the highest quality. They are very important tools for committees that guide policymakers, healthcare professionals, and researchers to make evidence-informed decisions. As compared to primary research, secondary research is easy to conduct and low cost because it involves only synthesizing what is already published. The number of systematic reviews in India now has increased compared to a few years back. Capacity building is something that we need in India for doing a systematic review. A requirement for that is Indian data for Indian problems. In terms of impactful trials for evidence synthesis for systematic review, we need to perform good clinical trials and do better reporting. A large trial does not necessarily mean good quality of evidence. The risk of bias needs to be assessed in the studies based on the systematic review. Suppose the study methodology is not of great quality. In that case, it will be excluded from the sensitivity analysis, which will further not be able to contribute to the evidence and decisions of the policymakers and healthcare professionals. The main goal is to evaluate the quality of evidence and make decisions based on that.
Finally, clinical trials can offer substantial benefits, and patients and care providers should actively look to enroll participants after understanding potential risks compared to existing treatment options. Policymakers should facilitate strong oversight of the trials and approval process and engage in active communication with care providers and patients/participants. There has to be a political will in terms of whatever development and scientific achievement that we are looking forward to increasing the “Impact of Clinical Trial.”
Acknowledgments
We want to acknowledge Dr. Niveditha Devisenapathy, Lead, Better Treatments, the George Institute for Global Health; Dr. Poongothai Subramani, Senior Scientist, Madras Diabetes Research Foundation; Dr. Aparna Mukherjee, Scientist, Indian Council of Medical Research, Dr. Mohammad Abdul Salam, Senior Research Fellow, the George Institute for Global Health for their insightful thoughts about the meaning of impactful clinical trials and efficient clinical research in the panel discussion of IHOPE 2023 conference along with Dr. Raja Narayanan, Principal Investigator, IHOPE, Network Director – Human Resources and Anant Bajaj Retina Institute Director – Suven Clinical Research Center, Consultant Ophthalmologist, Kallam Anji Reddy Campus, L.V. Prasad Eye Institute, Hyderabad and Dr. Mehul Mehta, Senior Medical Advisor, ASG and Instructor in Ophthalmology, Harvard Medical School.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- ICH harmonized tripartite guideline: Guideline for good clinical practice. J Postgrad Med. 2001;47:45-50.
- [Google Scholar]
- The CONSORT statement: Revised recommendations for improving the quality of reports of parallel-group randomized trials 2001. Explore (NY). 2005;1:40-5.
- [CrossRef] [PubMed] [Google Scholar]
- Multiply robust estimators of causal effects for survival outcomes. Scand Stat Theory Appl. 2022;49:1304-28.
- [CrossRef] [PubMed] [Google Scholar]
- The CONSORT patient-reported outcome (PRO) extension: Implications for clinical trials and practice. Health Qual Life Outcomes. 2013;11:184.
- [CrossRef] [PubMed] [Google Scholar]
- Strategies for participant retention in long term clinical trials: A participant-centric approaches. Perspect Clin Res. 2023;14:3-9.
- [CrossRef] [PubMed] [Google Scholar]
- Statistical principles for clinical trials. International conference on harmonisation E9 expert working group. Stat Med. 1999;18:1905-42.
- [Google Scholar]
- Sample sizes for clinical trials with normal data. Stat Med. 2004;23:1921-86.
- [CrossRef] [PubMed] [Google Scholar]
- Indian council of medical research's national ethical guidelines for biomedical and health research involving human participants: The way forward from 2006 to 2017. Perspect Clin Res. 2019;10:108-14.
- [CrossRef] [PubMed] [Google Scholar]
- practical guide to monitoring and management of clinical trials. 2002. Karger Medical and Scientific Publishers; Available from: https://edisciplinas.usp.br/mod/resource/view.php?id=2931506 [Last accessed on 2023 Dec 05]
- [Google Scholar]
- Clinical trials in India: Where do we stand globally? Perspect Clin Res. 2013;4:160-4.
- [CrossRef] [PubMed] [Google Scholar]
- Artificial intelligence and big data in healthcare. IHOPE J Ophthalmol. 2023;2:49-53.
- [CrossRef] [Google Scholar]






