Translate this page into:
Stem cell treatment for dry AMD

*Corresponding author: Rajani Battu, Eyestem Research Pvt Ltd, Bengaluru, Karnataka, India. rajani.battu@eyestem.com
-
Received: ,
Accepted: ,
How to cite this article: Narasimhaiah S, Prabhu R, Battu R. Stem cell treatment for dry AMD. IHOPE J Ophthalmol. 2024;3:32-8. doi: 10.25259/IHOPEJO_5_2024
Abstract
Age-related macular degeneration (AMD) is a leading cause of irreversible vision loss worldwide, affecting a significant proportion of the aging population. While wet AMD can be treated with anti-vascular endothelial growth factor injections, there is very little that can be offered to patients with dry AMD. This paper provides a review of AMD pathogenesis, current treatment methods for dry AMD, and the potential of cell-based therapies, particularly in the context of retinal pigment epithelium (RPE) replacement. AMD pathogenesis involves the formation of drusen impacting the health of the RPE. Aging, smoking, and genetic factors contribute to RPE dysfunction, leading to the progression of AMD. Newer therapies for dry AMD, including pegcetacoplan and avacincaptad pegol, mainly focus on reducing ongoing inflammation and addressing issues with complement regulation. This review discusses the emerging field of RPE replacement therapies, emphasizing the potential of induced pluripotent stem cells. Clinical trials involving RPE replacement therapy are discussed, showcasing ongoing efforts to develop effective treatments for dry AMD. While challenges, including immune rejection and integration issues, have to be solved, the potential benefits of RPE transplantation, either as cell suspensions or patches, remain significant. In conclusion, this paper highlights the promising prospects and challenges related to stem cell science in treating dry AMD, potentially marking a significant breakthrough in managing a previously untreatable disease.
Keywords
Age-related macular degeneration
Dry age-related macular degeneration
Stem cells
Induced pluripotent stem cells
Retinal pigment epithelium transplantation
INTRODUCTION
Age-related macular degeneration (AMD), a prominent cause of irreversible vision loss, primarily affects individuals over the age of 50 and accounts for 8•7% of all blindness worldwide.[1] The prevalence of AMD steadily increases with age, affecting 2% of the population at age 50 and one in four people by age 80.[2] According to the estimation by the World Health Organization, 20–25 million people suffer from AMD, out of which 8 million people have severe visual impairment.[3] Reports from India have shown a varied prevalence ranging from 1.8% to 47.8% of the population over the age of 60 years in different parts of India.[4,5]
AMD exists in two forms – “dry” and “wet” forms. Dry AMD is a chronic disease that usually causes some degree of visual impairment and may progress to severe blindness. In contrast, wet AMD affects only 10–15% of AMD patients and rapidly progresses to blindness if left untreated. Wet AMD is treated with injections of anti-vascular endothelial growth factors (VEGF) intravitreally. The end stage of dry AMD is referred to as “Geographic Atrophy” (GA). By definition, GA has been defined as a sharply delineated roughly round or oval area of hypopigmentation or depigmentation with increased visibility of the underlying choroidal vessels and of at least 175 µm in diameter on 30° or 35° color fundus photo images.[6] Vision deterioration in AMD can be attributed to either the macular loss of retinal pigment epithelium (RPE) and photoreceptors in its dry form, GA, or the infiltration of abnormal blood vessels into the RPE and/or retina, known as neovascular, exudative, or “wet” AMD, leading to choroidal neovascularization. While the initial lesions in GA may manifest beyond the fovea, allowing for seemingly normal central vision, the progression of vision loss becomes unavoidable. Once the central fovea is impacted, the rate of vision loss accelerates significantly.[7]
FUNCTION OF THE RPE CELLS IN A NORMAL EYE
The RPE functions as a continuous monolayer of hexanocuboidal post-mitotic epithelial cells. Connected to Bruch’s membrane and the choroid is the outer side of the RPE, while the outer segment of photoreceptor cells is connected to the inner side [Figure 1]. RPE sustains photoreceptor health by managing nutrient transport, helping outer segment renewal, protecting against light and oxidative stress, and maintaining retinal balance through signaling molecules.[8] RPE plays a key role in the visual cycle, supporting the isomerization of 11 cis-retinal to alltrans-retinal during the phototransduction cascade, as well as the recycling of all-trans-retinal back to 11 cis-retinal. It is also highly metabolically active since each RPE cell interacts with several photoreceptors. RPE contains two kinds of pigment: lipofuscin and melanin.[9] Melanin is an insoluble high-molecular-weight polymer derived from the enzymatic oxidation of tyrosine and dihydroxyphenylalanine and is present in membrane-limited granules in the RPE melanosomes. However, these melanosomes may differ from those in the RPE. Melanin in melanocytes originates from the neural crest, whereas the ones in RPE originate from the neural ectoderm. In aged RPE cells, melanin granules are frequently seen in association with lysosomes (melanolysosomes) and lipofuscin granules (melanolipofuscin).[8]
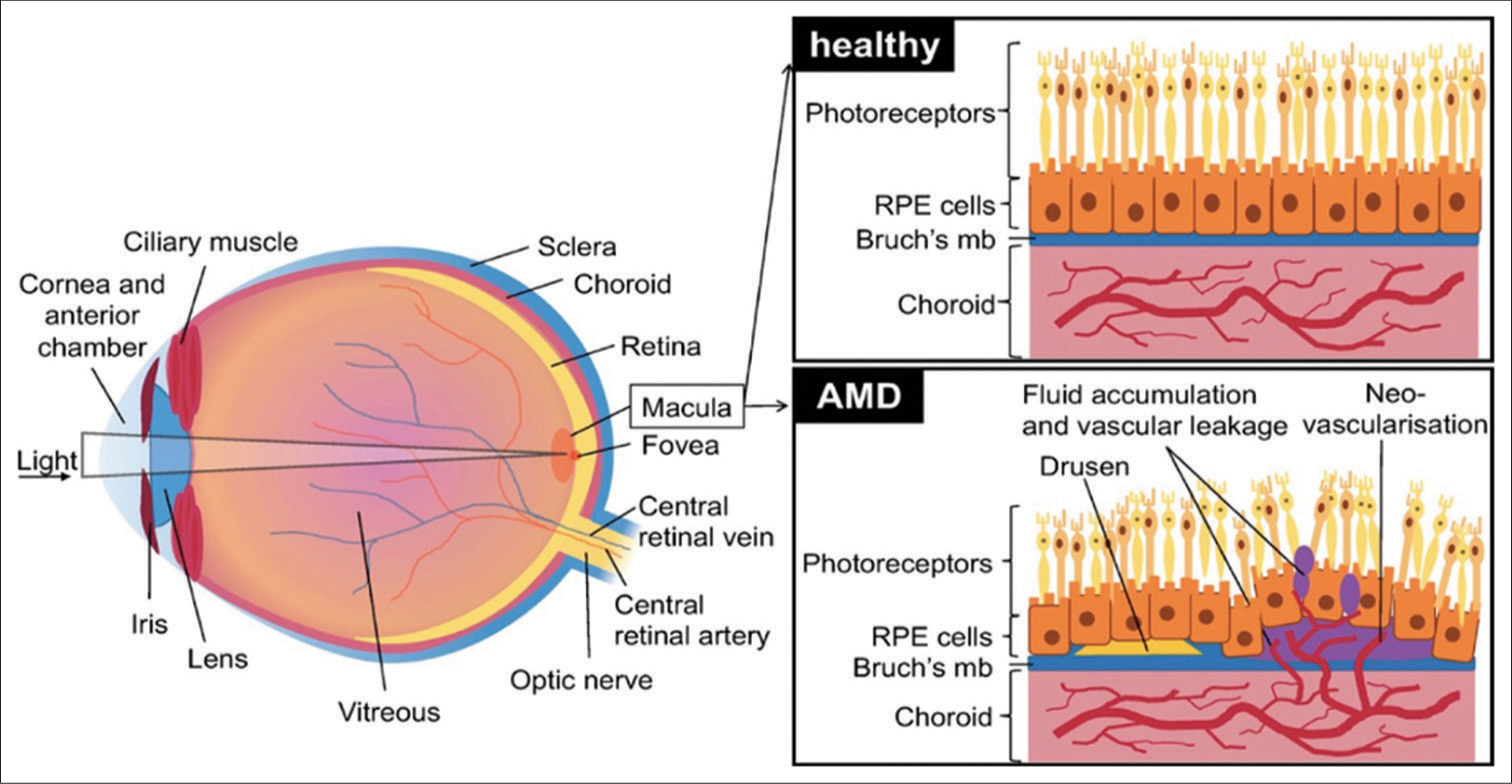
- Schematic of the eye showing healthy retinal pigment epithelium and changes in age-related macular degeneration. RPE: Retinal pigment epithelium, AMD: Age-related macular degeneration.
EMBRYOLOGICAL DEVELOPMENT OF RPE CELLS
The development of the human eye initiates at embryonic day (E) 18, marked by the formation of the visual groove at E22. This groove proceeds to sag, giving rise to the visual vesicle, which expands into the inner and outer layers of the optic cup. At around E30, the RPE layer begins to differentiate, and pigment particles appear in RPE cells by E35. Genes such as PAX6, LHX2, RAX, and SIX3, expressed in the neural plate before E8, play a crucial role in eye determination, eventually contributing to optic cup formation.[10]
In vertebrates, RPE cells typically derive from optic vesicles during embryonic development. Early optic vesicle cells possess bidirectional potential, capable of developing into either the retinal neurocortical layer or the RPE layer. Microenvironmental factors influence the fate determination and differentiation of RPE precursor cells.
During embryonic development, extracellular signals guide the differentiation process in a strict temporal and spatial order through the regulation of transcription factors and intracellular signaling pathways. Notably, the transcription factor microphthalmia-associated transcription factor (MITF) has been identified as a key player in normal RPE development. The knockout of MITF in mice has been shown to result in abnormal retinal development, underscoring its significance in the intricate process of eye development.[10]
ROLE OF RPE IN PATHOGENESIS OF AMD
The onset of AMD is identified by the presence of extracellular deposits rich in lipids and proteins resulting in the development of sub-RPE deposits, manifesting as distinct accumulations known as drusen. Drusen can exhibit either hard or soft texture or as continuous accumulations. The exact role of Drusen as the primary cause of RPE degeneration in AMD remains unclear. Nevertheless, they impact RPE health by hindering transport across Bruch’s membrane. In GA, there is a severe decline in vision due to the loss of RPE areas, photoreceptors, and the underlying choriocapillaris.[7,9] As the RPE ages, structural changes occur, such as melanin granule loss, increased residual body density, lipofuscin accumulation, basal deposits on Bruch’s membrane, drusen formation, Bruch’s membrane thickening, microvilli atrophy, and basal infoldings disorganization[11,12] [Figure 1]. While these changes are known, the underlying mechanisms are often unclear.
CURRENT TREATMENT OF DRY AMD
In AMD, chronic inflammation and dysregulation of the complement cascade contribute to the progressive degeneration of RPE cells and the subsequent loss of central vision. Pegcetacoplan and avacincaptad pegol represent novel therapeutic interventions designed to modulate complement activity and attenuate the detrimental effects of complement-mediated inflammation on RPE cells in AMD.
Pegcetacoplan, a pegylated form of a small molecule known as APL-2, is a C3 inhibitor that intervenes in the central hub of the complement cascade. By binding covalently to C3, pegcetacoplan prevents its cleavage into C3a and C3b, thereby inhibiting the formation of the C5 convertase and subsequent generation of the terminal complement complex. Pegcetacoplan exerts its action by specifically targeting and inhibiting complement component C3, a key mediator in both the classical and alternative complement pathways. By intercepting C3 activation, pegcetacoplan aims to mitigate the downstream pro-inflammatory and cytotoxic effects of the complement system on RPE cells, helping preserve retinal integrity and function.[13,14]
On the other hand, Avacincaptad Pegol, a fusion protein comprising a complement factor C5a receptor (C5aR) antagonist and the Fc region of an immunoglobulin, has a different approach to modulating the complement system. By competitively binding to C5aR, Avacincaptad Pegol interrupts the interaction between C5a and its receptor, thereby mitigating the inflammatory response associated with excessive complement activation. Developed to specifically bind and inhibit factor B, a crucial component of the alternative complement pathway, Avacincaptad Pegol disrupts the amplification loop of complement activation. This targeted intervention seeks to alleviate the burden of complement-mediated damage on RPE cells, thus slowing the progression of AMD.[15]
RPE REPLACEMENT AS THERAPY
RPE cells are pivotal in supporting photoreceptor function and in the phagocytosis and recycling of cellular debris, including drusen accumulation. Addressing the root cause of dry AMD has become a priority, emphasizing the replacement of degenerated or dysfunctional RPE cells. A promising approach involves harnessing the regenerative potential of stem cells, particularly induced pluripotent stem cells (iPSCs), to generate functional RPE cells for transplantation, this aims to restore the microenvironment crucial for retinal health.
The eyes, being relatively immune-privileged organs, present an ideal focus for stem cell therapy. Recently, researchers have established straightforward protocols for differentiating embryonic and iPSCs into RPE, opening up possibilities for treating diseases affecting the RPE, such as dry AMD, through stem cell therapy.
The utilization of stem cells in research and therapy has stirred controversy since the isolation of human embryonic stem cells (hESCs) in 1998 at the University of Wisconsin-Madison.[16] The creation of an immortal and pluripotent cell line from human blastocyst-derived cells marked a significant scientific advancement. However, ethical concerns arose due to the use of cells from human embryos. In 2001, the U.S. government suspended funding for creating further hESCs for research, limiting the available hESC lines for research. In 2006, Japanese researchers discovered iPSCs by transforming mouse fibroblasts into pluripotent stem cells. The subsequent generation of iPSCs from human cells was a major scientific breakthrough.[17]
iPSCs carry two significant advantages over embryonic stem cells (ESCs). First, since iPSCs are reprogrammed from mature adult somatic cells, the need for ESCs is eliminated. Second, iPSCs can be made autologously, meaning that each individual can theoretically have their own iPSC line. Disadvantages include a lower efficiency of reprogramming somatic cells into iPSCs, the introduction of mutations into target cells by transcription factors required for reprogramming, and the potential threat for tumor formation due to the expression of oncogenes triggered intentionally and unintentionally by viruses used to alter cells genomically.[18]
The RPE cells derived from hESCs or human-induced pluripotent stem cells are similar to human fetal RPE in terms of expression of key markers and RPE functionality, as demonstrated by phagocytosis assay and ion transport, and can rescue visual function.
PROTOCOL FOR GENERATION AND CHARACTERIZATION OF RPE
The pluripotent cells are differentiated into RPE by embryoid body (EB) aggregation [Figure 2]. These EBs are then coaxed into neuroectodermal fate by inhibition of pluripotency markers such as fibroblast growth factor 2 (FGF2) and bone morphogenetic protein 2/4 (BMP2/4) using dual suppressor of mothers against decapentaplegic (dual-SMAD) inhibition. All this is achieved using small molecules such as SB431542 and low-dose naltrexone (LDN). LDN acts as a bone morphogenetic protein (BPM) inhibitor, and SB431542 inhibits Activin/transforming growth factor beta (TGF-β) pathways. iPSCs differentiate to form the anterior neural plate and eye field specification to the formation of the bilayered optic cup. On the formation of the neuroectoderm, the non- rosette population gives rise to RPE. Mature RPE exhibits tightly packed cuboidal cells and blackish pigmentation with apical-basal polarity. ZO-1+ RPE cells indicate such tight junction and apical-basal polarity. They also express retinal transcription factors such as retinal homeobox protein (RX), cone-rod homeobox protein (CRX), and microphthalmia-associated transcription factor MITF.[19]
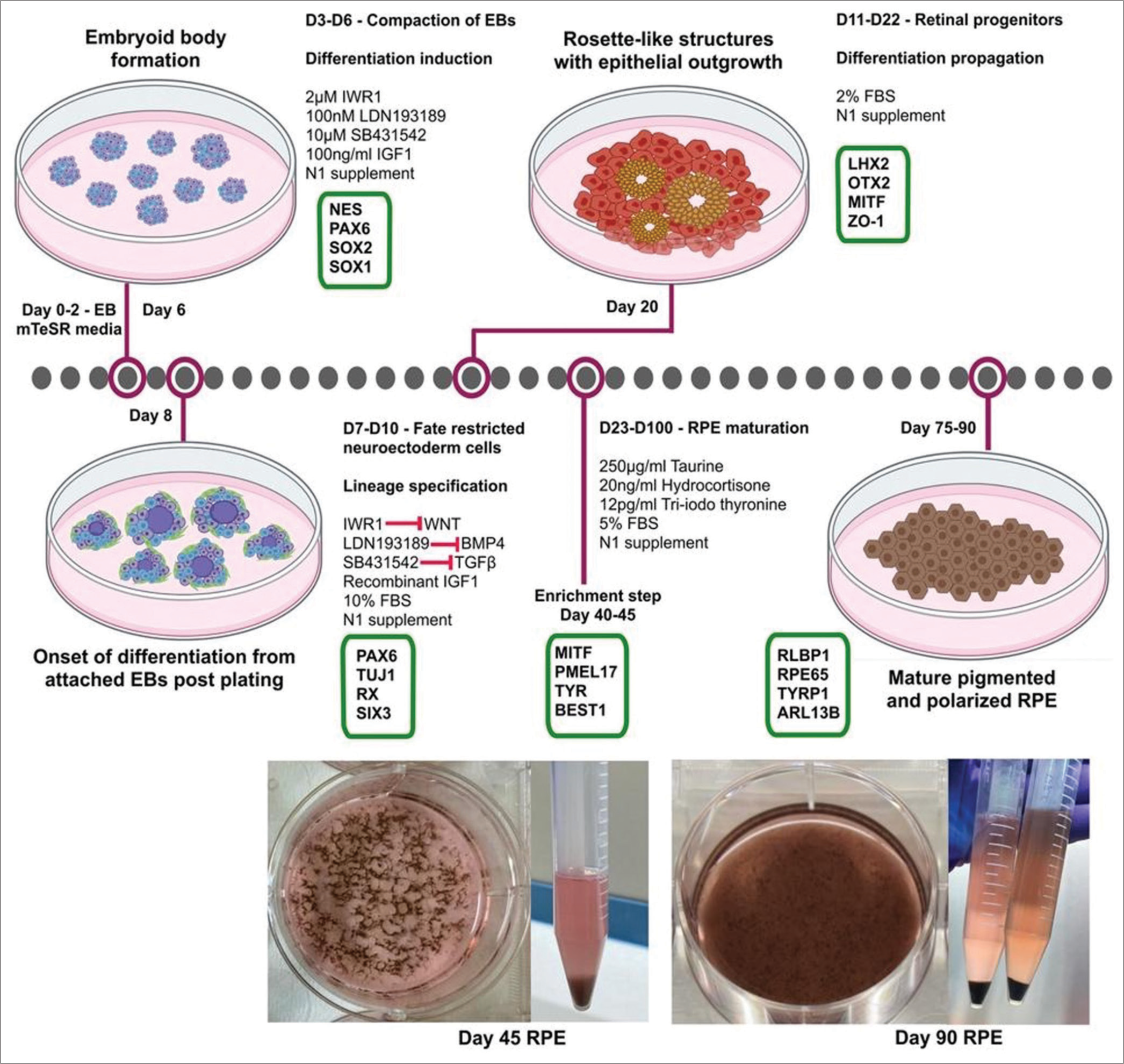
- Protocol for generation and characterization of human-induced pluripotent stem cell-derived retinal pigment epithelium. EB: Embyoid bodies, NES: Nestin, PAX6: Paired box-6, SOX2/1-Sex determining region Y-box2/1, FBSp: Fetal bovine serum, LHX2: LIM Homeobox 2, MITF: Microphthalmia-associated transcription factor, ZO-1: Zonula ocludens-1, mTESR: maintenance and expansion of stem cells, TUJ1: Class III beta tubulin, RX: Retinal homeobox protein, SIX3: Sine oculis homeobox 3, PMEL17: Pre melanosome protein 17, TYR: Tyrosinase, BEST1: Bestrophin 1, RLBP1: Retinaldehyde-binding protein 1, RPE65: Retinal pigment epithelium-specific 65 kDa protein, TYRP1: Tyrosinase related protein 1, ARL13B: ADP-ribosylation factor like protein 13B
RPE TRANSPLANTATION
RPE transplantation is an attractive target for cell therapy of dry AMD. The cell structure and function of RPE are fairly well-researched and understood. While RPE cells grow readily in laboratory cultures, they do not require forming synaptic connections to be functional. The number of cells required for transplantation is relatively small compared to other organs of the body. In addition, the outer retina can easily be imaged through optical coherence tomography and adaptive optics.[20] The rationale for using cell replacement therapy in AMD is twofold-one that the cells integrate with the host retinal tissue and show functional benefits, and the second is the benefit of neurotrophic factors associated with the transplantation of these cells.[21,22]
Cell therapy of AMD encompasses autologous and allogeneic approaches, each presenting distinct advantages and challenges. Autologous cell therapy involves transplanting a patient’s cells, minimizing immune rejection risk.[23] Harvesting RPE cells from the patient’s retina or obtaining iPSCs from peripheral blood for RPE differentiation are common methods, but challenges include obtaining sufficient cells from elderly patients. Allogeneic therapy, on the other hand, offers a potentially scalable and cost-effective solution using donor cells. However, concerns about immunogenicity and graft rejection necessitate the use of immunosuppressants or the use of immune-privileged cell sources to enhance graft survival.[24]
RPE administration primarily relies on two main approaches: injecting cell suspensions into the subretinal space and transplanting RPE patches. The use of cell suspension involves the injection of dissociated RPE cells directly into the subretinal space. This method capitalizes on the regenerative capacity of the introduced cells, aiming to restore the damaged RPE layer and improve overall retinal function. Cell suspensions offer advantages such as ease of administration, scalability, and the potential for widespread distribution within the retinal layers. However, challenges such as cell survival, integration, and controlled distribution within the targeted area must be carefully addressed to optimize therapeutic outcomes.[25]
In contrast, RPE cell patches involve the transplantation of engineered tissue constructs containing RPE cells. These patches, often composed of biocompatible scaffolds and cultured RPE cells, are strategically placed in the subretinal space. The cell patch approach offers a more organized and controlled method of delivering RPE cells, allowing for enhanced spatial precision and structural support. However, the fabrication and insertion of these patches demands meticulous attention to ensure cell viability, functionality, and seamless integration with the host tissue.[25]
The ultimate goal of RPE injections is to reconstruct the anatomic structure of the retina and rescue visual acuity by replacing the lost RPE cells in diseased retina. While engraftment of the transplanted RPE in the host is the mechanism of action behind long-term benefits, neuroprotective effects stimulated by grafted RPE cells are also substantially helpful. The RPE cells produce growth factors and cytokines through paracrine and autocrine signaling. Some cytokines, such as pigment epithelium-derived factor, interleukins, vascular endothelium growth factor, insulin-like growth factor, integrins, and metalloproteinase, may aid immune modulation or tissue regeneration. Similar neurotrophic and biological effects have been observed in patients with advanced AMD and Stargardt macular degeneration who were treated with MA09-hRPE.[26] It has also been demonstrated that when staying in the subretinal space, the non-integrated RPE stem-like cell may secrete large amounts of paracrine factors, particularly VEGF, to protect the retinal neurons from further degeneration in the short term.[27]
Intravitreal delivery of stem cells has been attempted using different types of stem cells, like adipose stem cells for the treatment of mild traumatic brain injury and bone marrow-derived mesenchymal stem cells for delivery of neurotrophic factors after optic nerve transection; however, they have shown very limited success.
CLINICAL TRIALS USING RPE REPLACEMENT THERAPY
Royal College of Surgeon Rats (RCS) with an inherited myeloid-epithelial-reproductive tyrosine kinase (MERTK) mutation serve as a good model for dry AMD. Experimental injection of healthy RPE cells into the subretinal space has shown preservation of the outer nuclear, outer plexiform, and photoreceptor layers and visual recovery.[28]
At present, there are several clinical trials in phase 1/2 for the transplantation of hESC/iPSC-derived RPE either as suspensions or sheets in patients with dry AMD and Stargardt macular degeneration.[29] Lineage cell therapeutics uses a unique method to deliver their cells in the sub-retinal space through the suprachoroidal approach through microinjection using the Orbit Subretinal Delivery System developed by Gyroscope Therapeutics (formerly Orbit Biomedical, Ltd.). Their cell therapy product, OpRegen, an allogeneic RPE cell therapy, is presently under scrutiny in Phase 2a clinical study (NCT02286089), where its effectiveness is being assessed in patients with GA secondary to dry AMD. This therapeutic advancement is the result of a collaborative effort between Lineage, Roche, and Genentech on a global scale, with exclusive involvement.[30]
Regenerative Patch Technologies has developed a composite subretinal implant that consists of a polarized monolayer of hESC-derived RPE (hESC-RPE) on a fragile, synthetic parylene substrate designed to mimic Bruch’s membrane, named the California Project to Cure Blindness-RPE 1.[21] Sharma et al. have developed an AMD-patient-specific iPSC-derived RPE (iRPE)-patch using a biodegradable scaffold that forms a monolayer of RPE cells in animal models when injected in the sub-retinal space.[31] Liu et al. from Singapore showed that RPE sphere-derived stem cells (SDSCs) obtained from mouse RPE cells transplanted under the macula of non-human primates was able to integrate with the host retina, recover RPE-specific markers, and support photoreceptor function. Since the majority of these are allogenic RPE transplants, it is necessary to use systemic or local immunosuppression.[27]
Eyestem Research in India is developing an allogenic suspension of RPE cells to be delivered in the sub-retinal space in patients with GA secondary to dry AMD. Pivotal efficacy and safety studies have been done at Oregon Health and Science University, Portland, and Dabur Research Foundation, New Delhi, and Phase 1/2a trials have been initiated.[19]
CHALLENGES AND LIMITATIONS
Cell-based therapies involving RPE cells show promise but face various scientific challenges and limitations. Obtaining a reliable source of functional RPE cells remains a hurdle, requiring advanced techniques for isolation, expansion, and differentiation. The quality and purity of RPE cell populations exhibit variability, impacting therapeutic efficacy. Ensuring the integration and long-term survival of transplanted RPE cells within the hostile microenvironment of the diseased retina is challenging, with potential issues of immune rejection and inflammatory responses compromising therapeutic outcomes. Moreover, immunogenicity concerns, especially with allogeneic sources, demand robust immune modulation strategies to prevent graft rejection and long-term complications. Achieving functional integration with the host retina and establishing optimal timelines for intervention add further complexity.[32]
Delivering a suspension of RPE cells into the subretinal space remains a surgical challenge. The traditional route involves pars plana vitrectomy followed by cell injection into the subretinal space via a small gauge needle but it often results in cellular efflux into the vitreous cavity. Suprachoroidal access to the subretinal space avoids retinotomy and associated complications but requires extensive training and poses risks of hemorrhage. Injecting an RPE sheet is even more challenging, given the requirement for a larger retinotomy and its attendant risk of complications. Further, injecting either a suspension or patch of RPE cells in an area of atrophic and thinned retina may increase the risk of surgical complications.
CONCLUSION
Although in its infancy, stem cell research is advancing and offers unprecedented opportunities for cell replacement, this is an attractive option since it overcomes the limitations of gene therapy and pharmaceutical therapy. Stem cell therapy and subretinal transplantation of RPE cells seem promising new ways to replace the lost cells, with several stem cell therapies on the anvil for hitherto untreatable diseases.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- The prevalence of age-related maculopathy by geographic region and ethnicity. Prog Retin Eye Res. 1999;18:371-89.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564-72.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for age-related macular degeneration: Findings from the Andhra Pradesh eye disease study in South India. Invest Ophthalmol Vis Sci. 2005;46:4442-9.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and predictors of age related macular degeneration in the population of Punjab: North Indian age related macular degeneration epidemiology and molecular genetic study (NI-ARMEMS) Int J Health Sci Res. 2018;8:108.
- [Google Scholar]
- An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv Ophthalmol. 1995;39:367-74.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanisms of age-related macular degeneration. Neuron. 2012;75:26-39.
- [CrossRef] [PubMed] [Google Scholar]
- The role of the retinal pigment epithelium: Topographical variation and ageing changes. Eye (Lond). 2001;15:384-9.
- [CrossRef] [PubMed] [Google Scholar]
- Dry age-related macular degeneration: Mechanisms, therapeutic targets, and imaging. Invest Ophthalmol Vis Sci. 2013;54:F68-80.
- [CrossRef] [PubMed] [Google Scholar]
- Functions and diseases of the retinal pigment epithelium. Front Pharmacol. 2021;12:727870.
- [CrossRef] [PubMed] [Google Scholar]
- Age-related changes in the retinal pigment epithelium (RPE) PLoS One. 2012;7:e38673.
- [CrossRef] [PubMed] [Google Scholar]
- Experimental models in neovascular age related macular degeneration. Int J Mol Sci. 2020;21:4627.
- [CrossRef] [PubMed] [Google Scholar]
- C3 inhibition with pegcetacoplan in subjects with paroxysmal nocturnal hemoglobinuria treated with eculizumab. Am J Hematol. 2020;95:1334-43.
- [CrossRef] [PubMed] [Google Scholar]
- Association of pegcetacoplan with progression of incomplete retinal pigment epithelium and outer retinal atrophy in age-related macular degeneration: A post hoc analysis of the FILLY randomized clinical trial. JAMA Ophthalmol. 2022;140:243-9.
- [CrossRef] [PubMed] [Google Scholar]
- Complement inhibitors for advanced dry age-related macular degeneration (Geographic atrophy): Some light at the end of the tunnel? J Clin Med. 2023;12:5131.
- [CrossRef] [PubMed] [Google Scholar]
- Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145-7.
- [CrossRef] [PubMed] [Google Scholar]
- Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861-72.
- [CrossRef] [PubMed] [Google Scholar]
- Stem cell-derived retinal pigment epithelium cell therapy: Past and future directions. Front Cell Dev Biol. 2023;11:1098406.
- [CrossRef] [PubMed] [Google Scholar]
- An improved protocol for generation and characterization of human-induced pluripotent stem cell-derived retinal pigment epithelium cells. STAR Protoc. 2022;3:101803.
- [CrossRef] [PubMed] [Google Scholar]
- Newer therapeutic options for inherited retinal diseases: Gene and cell replacement therapy. Indian J Ophthalmol. 2022;70:2316-25.
- [CrossRef] [PubMed] [Google Scholar]
- Patching the retina with stem cells. Nat Biotechnol. 2018;36:311-3.
- [CrossRef] [PubMed] [Google Scholar]
- iPSC-derived retina transplants improve vision in rd1 end-stage retinal-degeneration mice. Stem Cell Reports. 2017;8:69083.
- [CrossRef] [PubMed] [Google Scholar]
- Cell-based therapies for age-related macular degeneration. Adv Exp Med Biol. 2021;1256:265-93.
- [CrossRef] [PubMed] [Google Scholar]
- Current state of stem cell-based therapies: An overview. Stem Cell Investig. 2020;7:8.
- [CrossRef] [PubMed] [Google Scholar]
- Current treatment limitations in age-related macular degeneration and future approaches based on cell therapy and tissue engineering. J Ophthalmol. 2014;2014:510285.
- [CrossRef] [PubMed] [Google Scholar]
- Subretinal transplantation of embryonic stem cell-derived retinal pigment epithelium for the treatment of macular degeneration: An assessment at 4 years. Invest Ophthalmol Vis Sci. 2016;57:ORSFc1-9.
- [CrossRef] [PubMed] [Google Scholar]
- Paracrine effects of intraocularly implanted cells on degenerating retinas in mice. Stem Cell Res Ther. 2020;11:142.
- [CrossRef] [PubMed] [Google Scholar]
- Inherited retinal dystrophy in the RCS rat: Prevention of photoreceptor degeneration by pigment epithelial cell transplantation. Exp Eye Res. 1988;47:911-7.
- [CrossRef] [PubMed] [Google Scholar]
- Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt's macular dystrophy: Follow-up of two open-label phase 1/2 studies. Lancet. 2015;385:509-16.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical trial for [geographic atrophy] - Genentech a member of the Roche group. Available from: https://genentechclinicaltrials.com/en/trials/eye-disorder/damd/a-study-to-optimize-subretinal-surgical-delivery-and-to-78638.html [Last accessed 2024 Jan 25]
- [Google Scholar]
- Clinical-grade stem cell-derived retinal pigment epithelium patch rescues retinal degeneration in rodents and pigs. Sci Transl Med. 2019;11:eaat5580.
- [CrossRef] [PubMed] [Google Scholar]
- Challenges of cell therapies for retinal diseases. Int Rev Neurobiol. 2022;166:49-77.
- [CrossRef] [PubMed] [Google Scholar]







