Translate this page into:
Using artificial intelligence in diabetic retinopathy

*Corresponding author: Dr. Rajiv Raman, Shri Bhagwan Mahavir Vitreoretinal Services, Sankara Nethralaya, Chennai, Tamil Nadu, India. rajivpgraman@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Mohan S, Gaur R, Raman R. Using artificial intelligence in diabetic retinopathy. IHOPE J Ophthalmol 2022;1:71-8.
Abstract
Diabetic retinopathy (DR), a microvascular complication of diabetes, is a leading cause of blindness in India. Regular and timely screening for DR is recommended for the early diagnosis and appropriate treatment. However, mass screening for DR poses a significant challenge. Artificial intelligence (AI) is an important tool which has been used for diagnosing and grading diabetic retinopathy and aids in mass DR screening thus helping in faster and earlier screening of DR. This article aims to describe how AI is used in DR, software that are available for screening and the limitations and challenges in implementation of AI in health-care settings.
Keywords
Diabetic retinopathy
Artificial intelligence
Deep learning
Diabetic macular edema
Diabetic retinopathy screening
INTRODUCTION
Diabetes mellitus is a chronic non-communicable disease that can impact various organs in the body including the eye. Diabetic retinopathy (DR) is one of the complications of diabetes and a leading cause of visual impairment in India. Since it is asymptomatic in early stages with irreversible sight loss later, screening for DR is important to detect sight threatening disease and manage appropriately to prevent avoidable blindness. People with diabetes also require regular and repetitive annual retinal screening for timely detection of DR in addition to DM assessment. DR is clinically diagnosed through fundus examination or imaging methods such as fundus photography (FP) and optical coherence tomography (OCT). Mydriatic fundus examination for all patients with diabetes can be time-consuming. Fundus photos taken by digital cameras can be assessed by retina specialists which helps fasten the screening process, but this can also be time-consuming especially in countries with high incidence of diabetes. Early detection, diagnosis, and proper screening can decrease risk of visual loss to 57% and decrease overall cost of treatment.[1]
ARTIFICIAL INTELLIGENCE (AI)
AI, a concept first proposed in 1956 by McCarthy et al.,[2] is a system, in which machines mimic the cognitive function of the human mind. Machine learning is employed to generate and improve the machine’s ability to improve its own decision-making by learning from data provided to it. Deep learning (DL) is composed of algorithms that use a cascade of multilayered artificial neural networks to independently perform feature extraction from data.[3] Convolutional neural network (CNN) is a DL model suitable for processing images, and it is mainly composed of convolutional layers, pooling layers, and fully connected layers. Many studies haverevealed that AI has high sensitivity and specificity to recognize stages of DR from fundus photos.[4-6]
AI IN DR
The current utility of AI in DR is limited to preventive care, that is, in screening, with a rising interest in predictive features toward disease advancement and treatment burden. The American Diabetes Association has suggested that AI systems that detect more than mild DR and diabetic macular edema (DME) represent an alternative to traditional screening approaches.[7] These AI programs can also be applied on smartphone-based fundus cameras thus providing a low cost and effective method of screening for DR.[7] They have several advantages over human-based screening: they can grade thousands of images without fatigue, provide results within seconds to minutes, and reduce barriers to access in areas, where image graders/doctors are not present and decreased overall health burden.[3,8]
AI ALGORITHM DEVELOPMENT FOR DR
A good AI program needs to have an adequate balance between sensitivity and specificity. In DR screening, images of fundus are uploaded to the computer and DR lesions are detected by basic software. Depending on the system, the output is different DR present/absent, referable DR present/ absent, no DR/referable DR/Sight-threatening DR outcome, or others. There are many databases for screening of fundus photographs. The two major ones are Digital retinal images for vessel extraction and Messidor (Methods for evaluation segmentation and indexing techniques dedicated to retinal ophthalmology). Other databases include EyePacs, E-Ophtha, and Singapore Integrated DR program.[9] The dataset should be divided into training, validation, and test sets which should not overlap. The training set is to train the algorithm, the validation set is used for parameter selection and tuning, and the testing set is used to evaluate the actual performance of the AI system in clinical scenarios.[10]
[Figure 1] shows how a normal AI process works.
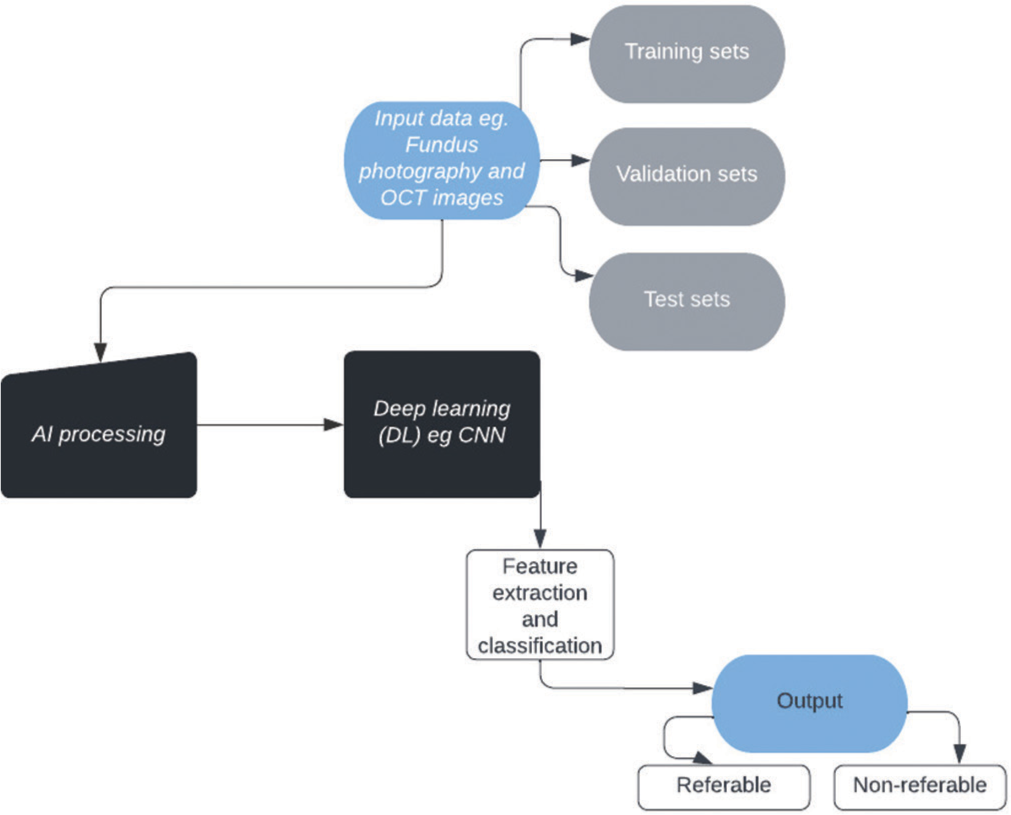
- Flow chart explaining the artificial intelligence process.
AVAILABLE AI SOFTWARE PROGRAMMES
IDx-DR was the first FDA approved AI software program which uses pictures from a non-mydriatic retinal camera (TRC-NW400, Topcon) and then uses an AI program to diagnose DR. The images taken are sent to a cloud-based server which utilizes the software and a DL algorithm to detect retinal findings in DR based on comparison with a large set of representative fundus images. If more than mild DR is detected, the patient is referred. If less than mild DR is detected, the patient is rescreened in 1 year.[7] It has shown good sensitivity and specificity. Abràmoff et al. reported that it had a sensitivity of 87.2% and specificity of 90.7% in detection of referable DR.[4] EyeArt by EyeNuk inc. was FDA approved in August 2020.[11] Rajalakshmi et al. validated a smartphone-based fundus photograph system for DR screening. They assessed the role of an automated AI algorithm for detecting DR and vision threatening DR. The EyeArt software was used to analyze images from dilated eyes. The results showed that the AI software exhibited 96% sensitivity and 80% specificity in detecting any DR and 99% sensitivity and 80% specificity in detecting vision-threatening DR.[5] Gulshan et al. from Google AI healthcare reported an AI system based on DL with excellent diagnostic capabilities called Inception V-3. A large training dataset of 128,175 images to test was used to validate the model. It shows a high sensitivity (>96%) and specificity (>93%) and area under the receiver operating characteristic curve (AUC) >0.99 in the external validation using two public databases. [6] Intelligent retinal imaging system (IRIS) is a type of AI system which is used an automated tele-retinal DR screening program which compared non-mydriatic fundus images with a standard data set from ETDRS and gave recommendations for referral, such as any patient with severe NPDR or more advanced disease. This program reported good sensitivity and a low false-negative rate.[12] The IRIS software is combined with Remidio’s handheld camera to provide instantaneous image gradeability. [Table 1] shows a list of some of the AI systems available with their sensitivities and specificities. Vision-threatening DR, for example, PDR and DME is important to identify as it requires prompt referral and management. DL algorithms have found reliability of grading up to 95% and sensitivities of 90.5–97% and the probability of missing severe NPDR, PDR, or macular edema is <1%. Roy et al. conducted the first pilot study in India which demonstrated the efficacy of automated DR imaging in screening studies in Indian population. Retmarker was used to analyze images of 1445 patients, of which images of 1207 patients had no evidence of DR (83.52%), which helped in initial screening of DR.[13] In a nationwide, DR screening program in Thailand DL versus human graders was compared for classifying DR. The study showed that relative to human graders, for detecting referable DR (moderate NPDR or worse), the DL algorithm had significantly higher sensitivity and a slightly lower specificity.[14]
| AI system | Authors | Algorithm | Type of camera | Mydriatic or non-mydriatic | AUC | Sensitivity | Specificity | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IDx-DR | Abràmoff et al.[4] | CNN | TRC-NW400, Topcon | Non-mydriatic | N/A | 87.2 | 90.7 | ||||||
| Van der Heijden et al.[22] | AlexNet, VGG net | TRC-NW400, Topcon | Non-mydriatic | 0.94/0.87 | 91/68 | 84/86 | |||||||
| Retmarker DR | Oliviera et al.[23] | Recognition of characteristic lesions | Cannon CR6-45NM fundus camera attached to a Sony power HD 3CDD digital color camera | Non-mydriatic | 0.849 | 95.8 | 63.2 | ||||||
| EyeArt | Solanki et al.[24] | Image analysis technology | Canon CR-2 AF | N/A | 0.941 | 93.8 | 72.2 | ||||||
| Rajalakshmi et al.[5] | Image analysis technology | Remidio fundus on phone (FOP), Remidio | Mydriatic | N/A | 99.3 | 68.8 | |||||||
| Bhaskaranand.[25] | Image analysis technology | Multiple fundus cameras | Both | 0.879 | 90 | 63.2 | |||||||
| Bhaskaranand.[26] | Image analysis technology | Multiple fundus cameras | Both | 0.965 | 91.3 | 91.1 | |||||||
| Gulshan et al.[6] | Inception V3 | 1st data set: Multiple cameras 2nd data set:Topcon TRC NW6 |
Both | 0.990–0.991 | 87.00–97.50 | 93.9–98.5 | |||||||
| Gulshan et al.[27] | Inception V4 | NM TRC, Topcon | Non-mydriatic | 0.963–0.980 | 88.90–92.10 | 92.20–95.20 | |||||||
| IDP | Abràmoff et al.[28] | Non-DL | Topcon TRC NW6 fundus camera+color video 3CCD camera | Non-mydriatic | 0.980 | 96.8 | 87 | ||||||
| Hansen et al.[29] | Non-DL | Topcon NW6S Fundus Camera | Mydriatic | 0.878 | 86.7 | 70 | |||||||
| Airdoc | He et al.[30] | Inception V4 | Topcon TRC-NW400 Fundus camera | Non-mydriatic | 0.95 | 91.8 | 98.79 | ||||||
| Huang et al.[31] | Inception V3 , SVM | 0.94 | 95.3 | 79.5 | |||||||||
| VoxelCloud Retina | Zhang et al.[32] | Inception-Res Net V2 | Multiple cameras | Non-mydriatic | N/A | 83.3 | 92.5 | ||||||
| VeriSee | Hseih et al.[33] | Inception V4, resnet | Canon CR-2 | Non-mydriatic | 0.95 | 89.2 | 90.1 | ||||||
| Eyegrader | Keel et al.[34] | Inception V3 | Digital Retinography System (DRS, CenterVue) | Non-mydriatic | 0.937–0.989 | 92.3 | 93.7 | ||||||
| PhelcomNet | Malerbi et al.[35] | CNN | Smartphone-based hand held devices | Mydriatic | 0.89 | 97.8 | 61.4 | ||||||
| Retianalyze Bosch DL | Bawankar et al.[36] | CNN | Bosch non-mydriatic fundus camera | Non-mydriatic | N/A | 91.18 | 96.91 | ||||||
| Singapore SERI NUS | Ting et al.[37] | VGGNet | Multiple cameras | N/A | 0.889–0.983 | 91.4–100.00 | 73.3–92.20 | ||||||
| EyeWisdom V1 | Zhang et al.[38] | Resnet-34, Inception V3 | Topcon TRC NW6S, Cannon CR2, KOWA Non-myd a-DIII 8300 | Non-mydriatic | 0.958 | 92.96 | 93.32 | ||||||
| Others | Gargeya and Leng.[39] | Data driven DL algorithm | Multiple cameras | N/A | 0.97 | 94 | 98 | ||||||
| Li et al.[40] | Inception-v3 | Multiple cameras | N/A | 0.955 | 92.5 | 98.5 | |||||||
| Cao et al.[41] | Bayesian model | Topcon TRC-NW6S/7S Fundus camera | Mydriatic | 0.938 | 94.9 | 92.8 | |||||||
| Sahlsten et al.[42] | Inception-v3 | Canon CR2 | Mydriatic | 0.987 | 89.6 | 97.4 | |||||||
| Krause et al.[43] | Inception-v3 | Centervue DRS, Optovue iCam, Canon CR1/ DGi/CR2, and TopconNWusing | N/A | 0.986 | 97.1 | 92.3 | |||||||
| Ultrawidefield imaging | |||||||||||||
| EyeArt | Wang et al. | Image analysis technology | Optos Daytona UWF system | Mydriatic | 0.873/0.851 | 91.7/90.3 | 50.0/53.6 | ||||||
| Nagawasa et al. | CNN | Optos Daytona UWF system | Mydriatic | 0.969 | 94.7 | 97.2 | |||||||
| Tang et al. | CNN | Optos Daytona UWF system | Mydriatic | 0.923–0.966 | 79.6–94.9 | 70.4–95.8 | |||||||
CNN: Conventional neural networks, UWF: Ultra wide field, N/A: Data not available
AI WITH OCT AND ULTRA-WIDEFIELD (UWF) IMAGING
Most current AI-based diagnostic systems are based on fundus photo. The disadvantage is that they can only recognize hard exudates in the posterior pole and may miss cases which have DME. OCT can also be used by AI for detecting of DME. Hassan et al. used both fundus and OCT images to achieve a sensitivity of 97% and specificity of 92% for referable DME detection. Zheng et al. devised an automated identification program DME examined by OCT which had good intraobserver and interobserver consistency. Lee et al. developed a CNN algorithm to detect intraretinal fluid on OCT. There are various other systems which combine OCT and AI techniques to detect DME. UWF imaging provides fast evaluation of non-mydriatic pupils to evaluate the peripheral retina which can recognize early damage of DR in the periphery. Wang et al. used a model with EyeArt software with an automated algorithm to detect referral warranting retinopathy with a sensitivity of 90.3%, specificity of 53.6%, and AUC of 0.851.[15] Nagasawa et al. also trained a CNN model to detect treatment naïve PDR in UWF images with a sensitivity of 94.7% and a high specificity of 97.2%, with an AUC of 0.969.[16] Tang et al. also validated a DL system which provided automated image quality assessment and detection of referable DR and vision-threatening DR from UWF-SLO images with high sensitivity and specificity.[17] DME is a common cause of visual impairment in diabetics and is treated with intravitreal anti-vascular endothelial growth factor (VEGF) injections. A major challenge associated with this treatment is determining an optimal treatment regimen and differentiating responders and non-responders to anti-VEGF. ML- or DL-based algorithms can be used to identify DME in fundus photo or OCT and predict patient’s response to an anti-VEGF agent. These AI-based prediction models can help in reducing the disease burden and offer the best line of treatment for the patient.[18]
AI IN REAL-WORLD SETTINGS
Adoption of AI technology is dependent on how it performs in the real-world clinical settings. Ruamviboonsuk et al. performed a prospective intervention cohort study to evaluate the real-world performance and feasibility of using a DL system into the healthcare system of Thailand. A total of 7940 patients were included for screening and 2412 patients were referred for DR, DME, ungradable images or low visual acuity. For vision-threatening DR, the deep-learning system had an accuracy of 94.7% (95% CI 93.0–96.2), sensitivity of 91.4% (87.1–95.0), and specificity of 95.4% (94.1–96.7) versus accuracy of 93.5%, sensitivity of 84.8%, and specificity of 95.5% in the retina specialist over-readers. This showed that a DL system can deliver real-time DR detection similar to retina specialists.[19] A computer-assisted customized algorithm was used for detection of DR in a tertiary eye care hospital in India which shows 78–79% sensitivity and 55–57% specificity in detecting DR. The algorithm was tested under physiological dilatation thus resembling real world settings.[20] Raman et al. proposed a step-wise algorithm for DR: From development to clinical use. This includes assessment of problem to be addressed by AI → Availability of data and data collection → Implementation costs → Deployment of AI in clinical settings → Clinical uptake → Maintenance over time. Each of these steps needs proper policies for useful functioning.[21]
LIMITATIONS AND CHALLENGES FOR AI IN DR
The very basis of building AI machines is that it learns from the information fed to it over the years by pattern recognition; however, there is lack of large databases of high-quality retinal images with proper annotations. Human graders are required to label and standardize images for further reference. This comes at a cost of time and manpower. There is also inter/ intra grader variability which is not accounted for. Further, AI algorithms are validated on this highly curated data which is not representative of real life screening scenarios.[44] There is a requirement for cameras with similar imaging systems and resolutions and pixels for large scale adoption along with image standardization. In ophthalmology in general, the ocular imaging consists of many different modalities such as color fundus photos, OCT, and Ultrasound images. Despite American academy of Ophthalmology and Asia pacific academy of ophthalmology guidelines, there is low compliance of ophthalmic imaging systems to adhere to the Digital Imaging and Communications in Medicine standards which are applied in radiological imaging.[45,46] At present, there is no easy way of sharing digital imaging data, and therefore, standardization of ophthalmic images is very central to what we indent to do with AI. With a few FDA approved machines and a few more being developed, another inherent hurdle is the comparison between different AI machines. This is due to lack of Key performance indicators. Different researchers have used different key indices to measure any AI model’s performance. Depending on the machine, the output might be different, for example, IDx –DR generates an overall per patient result, on the other hand, Retinalyse generates a per image result. Additional challenge in comparing the sensitivity and specificity of 2 machines is that studies use different cutoff values for Referable DR which may not always correspond with the same ETDRS grading levels.[44] Another confounding factor is demographic characteristics for instance many developers that have excluded individuals aged <40 years which risks the AI being unable to deal with highly reflective internal limiting membrane in young’s, hence decreasing the sensitivity of detecting referable DR in that age group.[6] AI opens many doors in its aim to provide better health facilities to the rural parts of the nation but it comes with its own cons. Like a lower specificity will identify more people without the disease who may in fact have changes of DR which will lead to a false sense of security to the people; on the other hand, a lower sensitivity may identify people with the disease who might not have it and that fails the entire purpose of building AI machines to decrease health burden. Downside of AI in DR so far is that it detects referable DR only by means of fundus photographs and macular optical tomography scans, but these machines should be made sensitive to other DR related changes like neovascularization of Iris or Angles. Therefore, algorithms based on comprehensive eye examination are required. At present, there are various screening programs already in practice such as computer programs, browser-based solutions, and even mobile applications which are already being used so it more on AI to integrate such programs and provide a more wholesome screening. In future, the results of artificial screening might surpass that of human graders, but can human graders be taken as an absolute objective truth? Some of the researchers, therefore, proposed patient outcome based truth rather than clinician’s agreement as an algorithm to build AI and showed that it works better than human graders.[4,28] There are considerable legal issues associated with development and implementation of AI algorithms such as product liability, medical malpractice, data security, and availability of consent, which can have serious implications. Developing countries economic burden in terms of cost of setting up the equipment which is in addition to the highly trained staff required to operate these equipment’s limits its use in already resource deprived parts of the nation. Several intergovernmental organizations and countries have proposed principles and guidelines for ethical use of AI, convergence was found on transparency, justice, fairness, non-maleficence, and responsibility. Several governments are introducing national policies and laws to govern the use of AI in healthcare. Organization for Economic cooperation and Development (OECD) launched a policy observatory in 2020 that “aims to help countries enable, nurture, and monitor the responsible development of trustworthy AI systems for benefit of society.” India is now the 27th member of this organization. AI is playing an ever expanding role worldwide and its alignment with regional policies, following rules and regulations adopted by OECD is the way forward.[47]
FUTURE OF AI IN DR
AI devices can provide screening decision without requiring a trained ophthalmologist. The integration of AI into healthcare will help in larger coverage for screening for DR. Following factors need to be considered in implementation of AI in healthcare settings: Policy setting which involves a risk adjust policy, technological implementation, and medical and economic impact quantification.[48] There are several challenges that stand in the way of wider adoption of AI. These include: Workflow integration, enhanced explainability and interpretability, workforce education on how to use AI, appropriate regulatory mechanisms, problem identification and focusing on intervention drive AI, understanding the potential impact of AI on clinician and patient relationship and data quality, access, and sharing and compliance with privacy. These factors are critical to implementation of AI in healthcare settings.[49] AI can ease the pressure on the healthcare system, particularly in nations with large spread and unequitable resources having high projected burden of diabetes. Automated DR screening methods can make the screening process more efficient, cost-effective, reproducible, and accessible.[50]
CONCLUSION
Almost 30–50% of individuals with diabetes do not adhere to screening recommendations and thus screening programs with non-mydriatic cameras can help in improving the screening process. AI technology can be used as for triaging to differentiate between urgent and non-urgent referrals. It also helps in the early detection of DR as screening can be conducted by all healthcare professionals and not just by the ophthalmologist. This helps in early and appropriate management, thus preventing loss of vision. Timely detection and early intervention are needed. The use of AI seems like a natural step in the future that can increase detection rates and reduce clinician’s burden at the same time. The aim is to reduce number of visits to an ophthalmologist, reduce overall cost of treatment, and optimize the number of patients needing referral.
However, it is important to realize that current medical knowledge is derived from decades of observational data gathering, hypothesizing, and validating the same by means of clinical research. Therefore, we cannot simply take a finding of AI without validating it to be consistent with our acquired medicine knowledge. To facilitate real word integration of AI programs, more studies are required to evaluate health professional’s acceptance and interpretability of AI to identify barriers to adoption and further to develop targeted solutions accordingly. The ultimate goal, here, is not replace a patient doctor relationship which is built on trust and compassion but to must complement it allowing quality medical services to people who need it the most.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Early detection of diabetic retinopathy based on deep learning and ultra-wide-field fundus images. Sci Rep. 2021;11:1897.
- [CrossRef] [PubMed] [Google Scholar]
- A proposal for the Dartmouth summer research project on artificial intelligence, August 31, 1955. AI Magazine. 2006;27:12.
- [Google Scholar]
- An Update on Artificial Intelligence for Detecting Diabetic Eye Disease: All Hype or the New Reality? Endocrinology Advisor. Available from: https://www.endocrinologyadvisor.com/home/topics/diabetes/ai-detection-of-diabetic-retinopathy-expert-perspective [Last accessed on 2022 Jun 12]
- [Google Scholar]
- Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Digit Med. 2018;1:39.
- [CrossRef] [PubMed] [Google Scholar]
- Automated diabetic retinopathy detection in smartphone-based fundus photography using artificial intelligence. Eye (Lond). 2018;32:1138-44.
- [CrossRef] [PubMed] [Google Scholar]
- Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316:2402-10.
- [CrossRef] [PubMed] [Google Scholar]
- Commissioner of the FDA Permits Marketing of Artificial Intelligence-Based Device to Detect Certain Diabetes-related Eye Problems. 2020. Silver Spring, Maryland: Food and Drug Administration; Available from: https://www.fda.gov/news-events/press-announcements/fda-permits-marketing-artificial-intelligence-based-device-detect-certain-diabetes-related-eye [Last accessed on 2022 Jun 12]
- [Google Scholar]
- Artificial intelligence for diabetic retinopathy screening: A review. Eye (Lond). 2020;34:451-60.
- [CrossRef] [PubMed] [Google Scholar]
- Retinal Image Databases-Medicmind. Available from: https://www.medicmind.tech/retinal-image-databases [Last accessed on 2022 Jun 12]
- [Google Scholar]
- Artificial intelligence for diabetic retinopathy. Chin Med J (Engl). 2022;135:253-60.
- [CrossRef] [PubMed] [Google Scholar]
- Health C for D and R. August 2020 510(K) Clearances Silver Spring, Maryland: Food and Drug Administration; 2020. Available from: https://www.fda.gov/medical-devices/510k-clearances/august-2020-510k-clearances [Last accessed on 2022 Jun 12]
- [Google Scholar]
- Evaluation of automated teleretinal screening program for diabetic retinopathy. JAMA Ophthalmol. 2016;134:204-9.
- [CrossRef] [PubMed] [Google Scholar]
- Automated diabetic retinopathy imaging in Indian eyes: A pilot study. Indian J Ophthalmol. 2014;62:1121-4.
- [CrossRef] [PubMed] [Google Scholar]
- Deep learning versus human graders for classifying diabetic retinopathy severity in a nationwide screening program. NPJ Digit Med. 2019;2:25.
- [CrossRef] [PubMed] [Google Scholar]
- Automated detection of diabetic retinopathy lesions on ultrawidefield pseudocolour images. Acta Ophthalmol. 2018;96:e168-73.
- [CrossRef] [PubMed] [Google Scholar]
- Accuracy of ultrawide-field fundus ophthalmoscopy-assisted deep learning for detecting treatment-naïve proliferative diabetic retinopathy. Int Ophthalmol. 2019;39:2153-9.
- [CrossRef] [PubMed] [Google Scholar]
- Detection of diabetic retinopathy from ultra-widefield scanning laser ophthalmoscope images: A multicenter deep learning analysis. Ophthalmol Retina. 2021;5:1097-106.
- [CrossRef] [PubMed] [Google Scholar]
- Narrative review of artificial intelligence in diabetic macular edema: Diagnosis and predicting treatment response using optical coherence tomography. Indian J Ophthalmol. 2021;69:2999-3008.
- [CrossRef] [PubMed] [Google Scholar]
- Real-time diabetic retinopathy screening by deep learning in a multisite national screening programme: A prospective interventional cohort study. Lancet Digit Health. 2022;4:e235-44.
- [CrossRef] [Google Scholar]
- Validation of a customized algorithm for the detection of diabetic retinopathy from single-field fundus photographs in a tertiary eye care hospital. Stud Health Technol Inform. 2019;264:1504-5.
- [Google Scholar]
- Using artificial intelligence for diabetic retinopathy screening: Policy implications. Indian J Ophthalmol. 2021;69:2993-8.
- [CrossRef] [PubMed] [Google Scholar]
- Validation of automated screening for referable diabetic retinopathy with the IDx-DR device in the Hoorn diabetes care system. Acta Ophthalmol. 2018;96:63-8.
- [CrossRef] [PubMed] [Google Scholar]
- Improved automated screening of diabetic retinopathy. Ophthalmologica. 2011;226:191-7.
- [CrossRef] [PubMed] [Google Scholar]
- Automated, high-throughput, image analysis for diabetic retinopathy screening. Invest Ophthalmol Vis Sci. 2015;56:1429.
- [Google Scholar]
- Automated diabetic retinopathy screening and monitoring using retinal fundus image analysis. J Diabetes Sci Technol. 2016;10:254-61.
- [CrossRef] [PubMed] [Google Scholar]
- The value of automated diabetic retinopathy screening with the eyeart system: A study of more than 100,000 consecutive encounters from people with diabetes. Diabetes Technol Ther. 2019;21:635-43.
- [CrossRef] [PubMed] [Google Scholar]
- Performance of a deep-learning algorithm vs manual grading for detecting diabetic retinopathy in India. JAMA Ophthalmol. 2019;137:987-93.
- [CrossRef] [PubMed] [Google Scholar]
- Improved automated detection of diabetic retinopathy on a publicly available dataset through integration of deep learning. Invest Ophthalmol Vis Sci. 2016;57:5200-6.
- [CrossRef] [PubMed] [Google Scholar]
- Results of automated retinal image analysis for detection of diabetic retinopathy from the Nakuru study, Kenya. PLoS One. 2015;10:e0139148.
- [CrossRef] [PubMed] [Google Scholar]
- Artificial intelligence-based screening for diabetic retinopathy at community hospital. Eye (Lond). 2020;34:572-6.
- [CrossRef] [PubMed] [Google Scholar]
- Artificial intelligence of diabetic retinopathy image recognition used in the real world. Technol Intell Eng. 2018;4:24-30.
- [Google Scholar]
- Artificial intelligence-enabled screening for diabetic retinopathy: A real-world, multicenter and prospective study. BMJ Open Diabetes Res Care. 2020;8:e001596.
- [CrossRef] [PubMed] [Google Scholar]
- Application of deep learning image assessment software veriseeTM for diabetic retinopathy screening. J Formos Med Assoc. 2021;120:165-71.
- [CrossRef] [PubMed] [Google Scholar]
- Feasibility and patient acceptability of a novel artificial intelligence-based screening model for diabetic retinopathy at endocrinology outpatient services: A pilot study. Sci Rep. 2018;8:4330.
- [CrossRef] [PubMed] [Google Scholar]
- Diabetic retinopathy screening using artificial intelligence and handheld smartphone-based retinal camera. J Diabetes Sci Technol. 2022;16:716-23.
- [CrossRef] [PubMed] [Google Scholar]
- Sensitivity and specificity of automated analysis of single-field non-mydriatic fundus photographs by Bosch DR Algorithm-comparison with mydriatic fundus photography (ETDRS) for screening in undiagnosed diabetic retinopathy. PLoS One. 2017;12:e0189854.
- [CrossRef] [PubMed] [Google Scholar]
- Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMA. 2017;318:2211-23.
- [CrossRef] [PubMed] [Google Scholar]
- The validation of deep learning-based grading model for diabetic retinopathy. Front Med (Lausanne). 2022;9:839088.
- [CrossRef] [PubMed] [Google Scholar]
- Automated identification of diabetic retinopathy using deep learning. Ophthalmology. 2017;124:962-9.
- [CrossRef] [PubMed] [Google Scholar]
- An automated grading system for detection of vision-threatening referable diabetic retinopathy on the basis of color fundus photographs. Diabetes Care. 2018;41:2509-16.
- [CrossRef] [PubMed] [Google Scholar]
- Artificial intelligence on diabetic retinopathy diagnosis: An automatic classification method based on grey level co-occurrence matrix and naive Bayesian model. Int J Ophthalmol. 2019;12:1158-62.
- [CrossRef] [PubMed] [Google Scholar]
- Deep learning fundus image analysis for diabetic retinopathy and macular edema grading. Sci Rep. 2019;9:10750.
- [CrossRef] [PubMed] [Google Scholar]
- Grader variability and the importance of reference standards for evaluating machine learning models for diabetic retinopathy. Ophthalmology. 2018;125:1264-72.
- [CrossRef] [PubMed] [Google Scholar]
- Reproduction study using public data of: Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. PLoS One. 2019;14:e0217541.
- [CrossRef] [PubMed] [Google Scholar]
- Recommendations for standardization of images in ophthalmology. Ophthalmology. 2021;128:969-70.
- [CrossRef] [PubMed] [Google Scholar]
- Ocular imaging standardization for artificial intelligence applications in ophthalmology: The joint position statement and recommendations from the Asia-Pacific academy of ophthalmology and the Asia-Pacific ocular imaging society. Asia Pac J Ophthalmol. 2021;10:348-9.
- [CrossRef] [PubMed] [Google Scholar]
- Ethics and Governance of Artificial Intelligence for Health. Available from: https://www.who.int/publications-detail-redirect/9789240029200 [Last accessed on 2022 Aug 12]
- [Google Scholar]
- Success factors of artificial intelligence implementation in healthcare. Front Digit Health. 2021;3:594971.
- [CrossRef] [PubMed] [Google Scholar]
- Artificial Intelligence in Health Care-the Hope, the Hype, the Promise, the Peril. Nasjonalt Senter for e-Helseforskning. Available from: https://ehealthresearch.no/rapporter/andre/artificial-intelligence-in-health-care-the-hope-the-hype-the-promise-the-peril [Last accessed on 2022 Aug 12]
- [Google Scholar]
- Fundus photograph-based deep learning algorithms in detecting diabetic retinopathy. Eye (Lond). 2019;33:97-109.
- [CrossRef] [PubMed] [Google Scholar]






